At Aurigene Pharmaceutical Services, we offer a wide range of integrated discovery biology services to meet your drug discovery needs. We offer end-to-end services including in vitro biology, ADME & DMPK studies, Toxicology, in vivo pharmacology and microbiology testing.
We provide integrated and standalone drug discovery services ranging from target identification to candidate nomination and further extension up to development services. Our best-in-class discovery biology services are supported by AAALAC accredited vivarium, BSL-2 compliant labs, in vivo facilities, GLP and other regulatory bodies accredited toxicology labs. Our discovery biology team offers a blend of scientific skills and pharmaceutical industry expertise with a proven track record of complex problem-solving capabilities. Biologists holding Masters, PhDs, and Postdocs are trained to undertake the most demanding drug discovery biology projects.
During the past two decades, the team in collaboration with our clients has been able to apply differentiated solutions to each aspect of drug discovery research, which has resulted in the discovery of many clinical candidates. Numerous research publications and feedback from clients are a testimony of our success.






In vitro Biology
Our team provides a wide range of services in Biochemical assays, Mechanistic assays, Functional assays, Immunological assays and various customized assays.
Drug Metabolism and Pharmacokinetics (DMPK)
We provide precise data and insightful analysis of a drug's absorption, distribution, metabolism and excretion (ADME) properties. These insights help the clients to shortlist compounds with a high likelihood of success in later stages of drug development. DMPK tests include:
- Physicochemical Parameters
- Metabolism Studies
- Permeability
- Distribution
- Drug-drug Interactions
- Pharmacokinetics
- Bioanalysis
In vivo Pharmacology Studies
Our in vivo pharmacology team has expertise across multiple therapeutic areas. The team is supported by an AAALAC accredited, GLP-certified vivarium and experienced scientists who can set up diverse efficacy models as per program/client needs. We offer target engagement and efficacy models for:
- Oncology and Immuno-oncology
- Metabolic Disorders
- Inflammation
- Pain
- Acute Inflammation
- Auto-immune Disorders
- Syngeneic Tumor
- Muscle Regeneration
- Skin Disorders
These are validated with reference compounds and supported by histopathology and biomarker analysis.
Pharmaceutical Microbiology Testing
Our pharmaceutical microbiology testing department has a BSL-2 compliant lab and a dedicated in vivo facility for bacterial and fungal infection murine disease models. Our team has vast experience in anti-infective drug discovery biology projects. The team has worked on several collaboration projects with innovator pharma companies to discover antibacterial and antifungal agents. We have also established in-vitro time-kill kinetics bioequivalence assays of generic products for regulatory submission.
Toxicology
We offer a full range of exploratory and GLP toxicology studies supported by clinical and anatomic pathology services, formulation analysis, toxicokinetic analysis, and interpretation and reporting. General toxicology studies range from single-dose and repeat dose toxicology studies, genetic toxicology, and IND and NDA enabling safety toxicology packages. We have a track record of successfully filing INDs using the in-house data with US FDA, MHRA, EMA, DCGI and ANDAs.
Why Aurigene Discovery Biology Services?
Expert team of scientists
65+ IDD projects delivered
State-of-the-art facilities
USFDA inspected labs with zero 483 observations
AAALAC accredited Animal House
NGCMA accreditation
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
OCTOBER 01, 2024
PROTACs: Research for a life without cancer
PROTACs: Proteolysis-targeting chimeras (PROTACs) are a rapidly evolving field with promising applications in cancer, neurodegenerative diseases, and other conditions where the regulation of protein levels is crucial. PROTACs are a novel class of small molecules designed to target specific proteins for degradation by the ubiquitin-proteasome sys...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Case study: Tackling CYP 2C9 inhibition challenges
The Problem: Active compounds in a project were found to be highly potent inhibitors of CYP 2C9 The compounds selectively inhibited CYP 2C9 with IC50 values <100 nM There was no considerable inhibition of the other CYP isoforms Our Mitigation Approach: CYP 2C9 inhibition data was generated for a larger set of co...
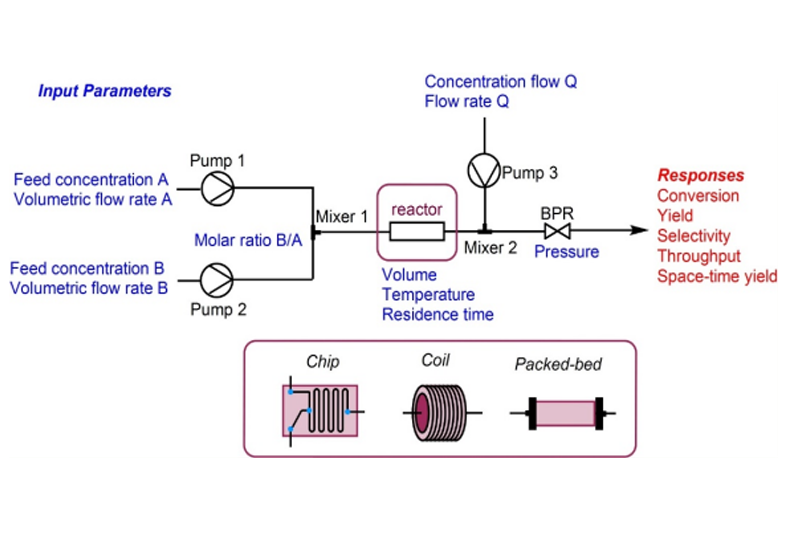
Read MoreSynthesis of Anti-covid Drug Nirmatrelvir Using Flow Chemistry
2022
Synthesis of the anti-covid therapeutic Nirmatrelvir by using flow chemistry to enhance efficiency of amide to nitrile conversion in a functionally and Stereochemically Embellished environment. ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market