Improvements in physicochemical properties can be achieved by altering the physical forms of a given compound such as polymorphs, solvates, amorphous, salts, cocrystals, and/or hydrates, etc.
At APSL, we have over 20 years of experience in solid state science including polymorph screening and development, salt screening, enantiomeric screening, X-ray diffraction, crystallization, and amorphous dispersions.






Solvent screening
- Solvent screening
- Polymorph studies will be performed with the API material
- Solvent screening will be performed with the below solvents - ICH-3: Ethyl acetate, n- Butyl acetate, IPA, MTBE, MIBK, Acetone, Heptane, n-Butanol, IPAC and 2- Methyl THF, DMSO etc.
ICH-2: Acetonitrile, Dichloromethane, Hexane, Methanol, Tetrahydrofuran, Toluene, 1, 4-Dioxane, Di –Isopropyl ether, cyclohexane etc. - Detailed solvent screening at different temperatures to classify the solvents for Anti and co-solvents
- Solubility curve generation, identification of Metastable Zone Width (MSZW)
- The solubility studies are intended to be performed with ~95% pure material
Studies for polymorph screening and crystallization
Crystallization experiments would be attempted to understand the process conditions to get the different morphs. It could be either solvated, non-solvated, hydrated, or hemihydrate. Various crystallization techniques will be used for screening the solid forms:
- Cooling crystallization
- Anti-solvent crystallization
- Seeding crystallization (Seed point evaluation)
- Evaporative crystallization
Polymorph-slurry mediated transformations
Various forms of the test compounds will be subjected to slurry-mediated transformation studies in organic solvents which are having optimum solubility -
- Slurring media, volume, and solid content will be appropriately adjusted to make sure that always excess solid is present.
- Time points of the slurry experiments can be decided as per requirements. Solid forms that remained after slurring either in organic solvents or aqueous phase mixtures will be separated and will be subjected to solid-form characterization (PXRD, DSC, and TGA).
Polymorph or crystallization process optimization
- Crystallization process will be optimized with PAT like FBRM (Focused Beam Reflectance Measurement) which shows online particle counts and chord length distributions of the particles and PVM (Particle Vision Microscope) which shows online particle shape
- Process finalization to achieve desired yield and quality parameters
- ATFD studies if required, in case of amorphous forms and heat-sensitive materials
Optimization of filtration, drying and particle size distribution
- Filtration and drying studies from a scale-up point of view optimize the conditions
- Particle size distribution & milling studies shall be performed based on the requirement
- Stress studies for solids (polymorph stability with milling and micronation)
- We have all the associated lab and plant infrastructure with multi-mills, centrifuges, micronizes, and ANFDs
Why Aurigene Crystallization and Polymorphism Services?
Expertise and experience of 20+ years
Comprehensive infrastructure at lab and plant for a full suite of services
State-of-the-art PAT tools, and QbD approach to support optimization
Phase-appropriate development approach
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
OCTOBER 01, 2024
PROTACs: Research for a life without cancer
PROTACs: Proteolysis-targeting chimeras (PROTACs) are a rapidly evolving field with promising applications in cancer, neurodegenerative diseases, and other conditions where the regulation of protein levels is crucial. PROTACs are a novel class of small molecules designed to target specific proteins for degradation by the ubiquitin-proteasome sys...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Case study: Tackling CYP 2C9 inhibition challenges
The Problem: Active compounds in a project were found to be highly potent inhibitors of CYP 2C9 The compounds selectively inhibited CYP 2C9 with IC50 values <100 nM There was no considerable inhibition of the other CYP isoforms Our Mitigation Approach: CYP 2C9 inhibition data was generated for a larger set of co...
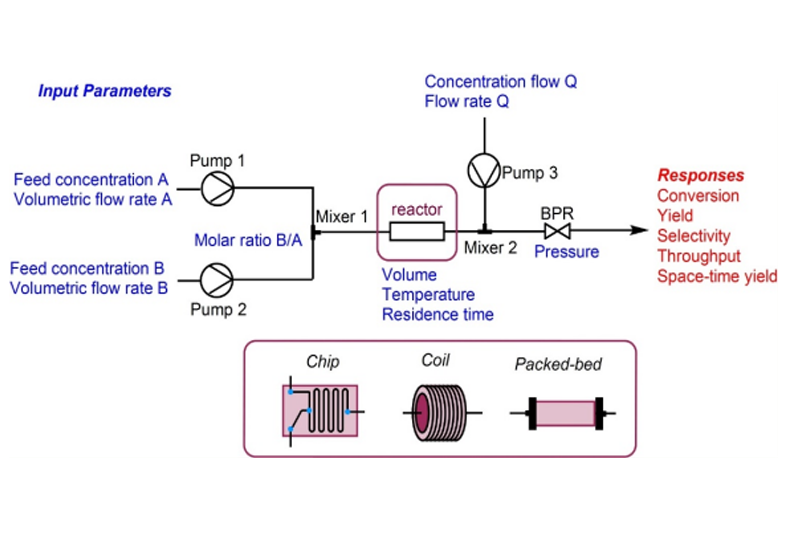
Read MoreSynthesis of Anti-covid Drug Nirmatrelvir Using Flow Chemistry
2022
Synthesis of the anti-covid therapeutic Nirmatrelvir by using flow chemistry to enhance efficiency of amide to nitrile conversion in a functionally and Stereochemically Embellished environment. ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market