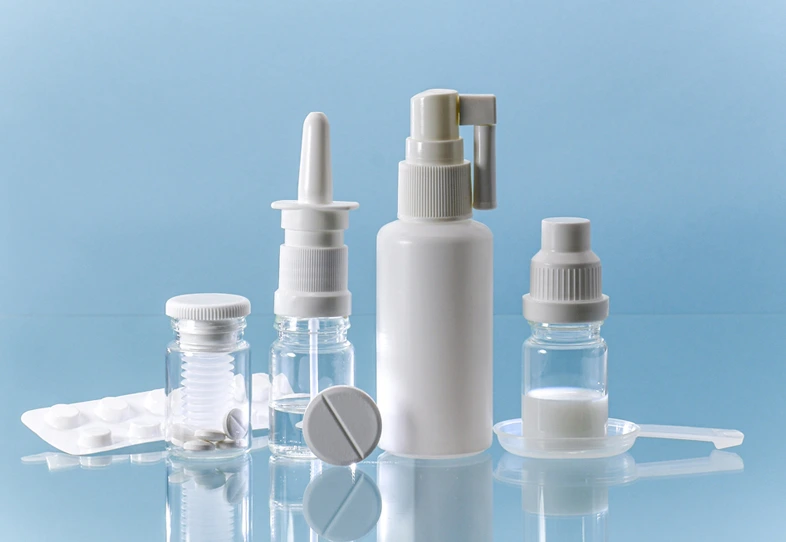
We offer drug product development through a highly skilled scientific team with expertise in variety of dosage forms like oral, parenteral and topical. We specialize in pediatric dosage forms, fixed dose combinations and modified-release dosage forms. We also support for development of customized formulations for animals. Our team has extensive experience in developing complex formulations and provide tailored solutions to optimize the development time and costs.
Our team is committed to delivering quality results in a time-bound and cost-effective manner at each stage of the development process. Customers also select us, to evaluate container closure systems, storage, and transportation conditions.
ICH-compliant stability studies along with a range of conditions to cover various climatic zones across the globe, help in making decisions early in the drug development cycle.
With decades of experience in formulation development, our scientists look forward to accelerating the time to market of your molecule.



Solid Orals
- mmediate-release tablets (chewable tablets, orally disintegrating tablets, sublingual tablets and bi-layer tablets)
- Modified release tablets (Monolayer, bilayer and mini tablets)
- Fixed dose combinations (Monolayer, Bilayer and Multi particulate systems (Granules, pellets or mini-tablets and combinations thereof)
- Hard Capsules (Hard gelatin or Cellulose capsules with powder in capsule, pellets in capsules) and soft gelatin capsules
- Multi-particulate systems (Pellets and mini tablets)
- Powder in sachet for solution/suspension
Oral Liquids
- Oral solutions with dosing cup
- Oral drops with dosing device
- Oral suspension
- Oral emulsions
Parenterals
- Liquid injectables
- Lyophilized injectables
- Complex injectables
- Ophthalmic and otic solutions
- Nasal solutions ( Sprays and Blow-Fill-Seal)
Topical
- Ointment
- Cream
- Gel
Why Aurigene Drug Product Development Services?
Services across the product lifecycle
USFDA inspected lab and manufacturing facilities
Experience with advanced formulation technologies
20+ years of experience in formulation
Other Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources

JANUARY 04, 2021
Application & Manufacturing of High Potent Active Pharmaceutical Ingredient (HPAPI)
High Potent Active Pharmaceutical Ingredient (HPAPIs) are majorly used to treat oncology-related conditions. Usually, a compound is considered high-potent when the operational exposure limit is below 10 µg/m3 of air as an 8-hour time-weighted average. High Potent drugs can be administrated through oral, injection or topical route depending on the s...
Read More
Peptide Development and Manufacturing
Peptide Development and Manufacturing Peptides are short chains of amino acids that are linked by peptide bonds. Several peptides linked together are called polypeptides. A protein contains one or more polypeptides. Therefore, proteins are long chains of amino acids held together by peptide bonds. ...
Read More
Methoxy Polyethylene Glycol (m-PEGs)
Aurigene Pharmaceutical Services is a leader in the synthesis of activated MethoxyPolyethyleneGlycol(m-PEGs), With a comprehensive product range and customized services. ...
Read More
Technology meets sustainability - How a complex API development process goes green
We are always looking for ways to enhance the sustainability of our products and services. Our team successfully developed a scalable manufacturing process for the API product of one of our Biotech clients using eco-friendly manufacturing technologies. Read the case study to learn more. ...
Read MoreConstruction of a six-membered fused N-heterocyclic ring via a new 3-component reaction: synthesis of (pyrazolo)pyrimidines/pyridinesw
2012
A conceptually new three-component reaction was developed to construct a six-membered fused N-heterocyclic ring affording (pyrazolo)pyrimidines/pyridines as potential inhibitors of PDE4. The reaction is catalyzed by triflic acid in acetic acid in the presence of aerial oxygen. ...
Read More-
Identification of Degradants of Thermal and Oxidation Stress Studies of Empagliflozin and Linagliptin Tablets by HPLC-PDA and LC-MS Instrumental Techniques
2005
Objective of the manuscript is to identify the degradants observed in the thermal and oxidation degradation sample of Empagliflozin and Linagliptin tablets by using LC-MS and HPLC-PDA instrumental techniques. Thermal and oxidation degradation samples were injected in HPLC-PDA and LC-MS instruments. Mass of the degradants were detected by LC-MS technique, ...
Read More -
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market