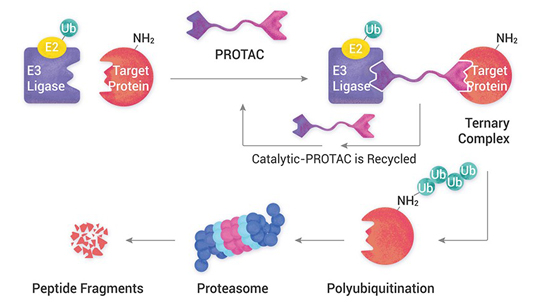
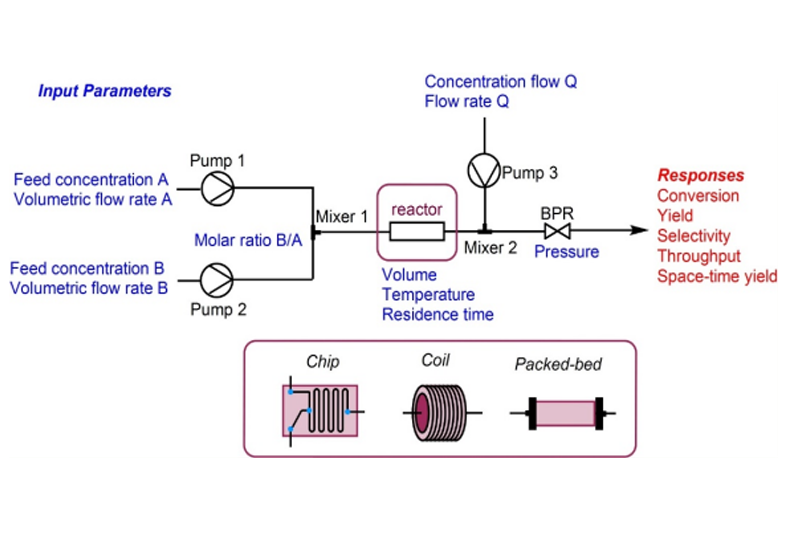
We support and offer a diverse range of services in the areas of:
Recombinant DNA technology Antibody gene sequencing Mutagenesis Library generation Plasmid DNA preparation Microbial genome engineering Gene expression analysis and IVT mRNA synthesis
Infrastructure and facilities
Our state-of-the-art laboratory equipped with high-end instrumentation and the experienced IT and engineering teams support discovery and development services under biology capabilities.
Real-Time PCR, Gradient PCR instruments, GeneAmp PCR system Gel documentation systems Nanodrop spectrophotometer Pulsed field electrophoresis Benchtop fermenters Biosafety cabinets- BSL I & II Static incubators and shakers Wave bioreactor Flow cytometer LiCoR imaging system Biorad molecular imager Nikon Eclipse E800- inverted light microscope Shimadzu UV spectrophotometer Beckman DU 640 spectrophotometer Perkin Elmer Victor X5 2030 multilabel reader Biotek microplate reader Photomultiplier detection system



Recombinant DNA technology
Cloning and vector generation:
Gene cloning service at APSL supports customized generation of recombinant constructs by various methods for generation of antibodies, enzymes, and reagent proteins:
- Ligation dependent and independent cloning
- Recombination based high throughput cloning
- De novo cloning (synthetic gene fragments, PCR, and cDNA based)
- Customized vector construction based on client requirement
Plasmid DNA preparation:
Plasmid DNA purification service caters to both small research facilities as well as large-scale manufacturing for biotech and pharmaceutical companies:
- Research grade plasmid at small scale suitable for cloning, mutagenesis, microbial transformation, and protein production
- High-quality industrial grade plasmid suitable for :
- protein
- antibody production
- stable cell line generation in mammalian cells
- bacmid generation for protein expression in insect cells
- viral packaging
- in vitro mRNA synthesis
- vaccine
- gene therapy studies, and
- animal immunization
- Process development for customized plasmid propagation and purification
Mutagenesis:
Site-directed mutagenesis service enables life science researchers by creating specific DNA mutations for studying gene regulation, DNA-protein interactions, protein structure/function relationship, enzyme function, and novel recombinant proteins:
- Point mutation, insertions, deletion, or gene fragment replacements
- Customized mutagenesis requirements such as random and site-directed mutagenesis and mutant library generation.
- Gene shuffling, combinatorial mutagenesis, alanine scanning, and site saturation mutagenesis
Antibody gene sequencing and reformatting
Antibody sequence information from hybridoma is essential for protection, preservation, and optimization of monoclonal antibodies (mAbs). Antibody gene sequencing and reformatting at APSL is a one-stop solution for recombinant expression and production of antibodies:
- RACE (Rapid Amplification of cDNA Ends) using template switching adapter ligation
- Degenerate oligos
- De novo antibody sequencing and analysis using LC-MS/MS
- Reformatting antibody gene sequences into various other production formats like bispecifics, mAb, scFv, Fab, F (ab)'2, and VHH/nanobody
- Antibody gene sequencing for mouse, rat, rabbit and other species.
Strain engineering
Experience in genome scale modification of bacterial and yeast strains for production of recombinant proteins, platform chemicals, and metabolic engineering:
- BSL-1 and BSL-2 microbes
- Gene knock out and knock-in using homologous recombination based methods
- Characterization of engineered strains using microbiological methods, RT-PCR, and advanced analytics
- CRISPR-Cas9 based mutagenesis
Library generation and evaluation
Constructing cDNA libraries, phage libraries, and yeast libraries along with provisions for high throughput screening and functional characterization.
Gene expression analysis
Regulation and expression of genes at the transcriptional and translational stages play a crucial role in determining gene function. Scientists at APSL carry out routine expression studies by several methods such as:
- Quantitative real time PCR
- ELISA in various formats
- Flow cytometry
- Western blotting
- Mass spectrometry
Residual protein, DNA, and Mycoplasma detection
Residual host-cell proteins (HCP) and host-cell DNA (HCD) are impurities that remain in recombinant bio therapeutics. We ensure that these impurities and contaminants are detected and reduced to levels below those guided by regulatory agencies:
- Quantitative real-time PCR
- ELISA
- SPR (Biacore)
- Mycoplasma test
Why Aurigene Molecular Biology Services?
State-of-the-art facilities with high-end instrumentation
Wide range of tools under one umbrella
Small-scale and large-scale manufacturing
Customized generation of recombinant constructs
Fast and innovative solutions
One-stop solution for recombinant expression and production of antibodies
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
FEBRUARY 25, 2025
Transforming Drug Discovery with Aurigene.AI
In the early 2000s, developing Sovaldi, a hepatitis C treatment, took over a decade and nearly $2 billion. Similarly, Zolgensma, a gene therapy for spinal muscular atrophy, required 15 years due to its complexity. However, the advent of artificial intelligence (AI) has revolutionized drug discovery. For example, in 2022, Pfizer's PAXLOVID, an oral COVID...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read MoreFamiliarization, process optimization, and cGMP manufacturing and supply of 30.0 kg of a Bioactive Nucleotide (NAD Booster)
Background: A US-based biopharmaceutical company approached Aurigene Pharmaceutical Services for the familiarization, process development, and cGMP manufacturing and supply of 30.0 kg Nucleotide product (NAD booster) for phase-appropriate studies. The synthesis of the desired product involves three linear stages, which starts with reaction of a pentose...
Read MoreIdentification of Degradants of Thermal and Oxidation Stress Studies of Empagliflozin and Linagliptin Tablets by HPLC-PDA and LC-MS Instrumental Techniques
2022
Objective of the manuscript is to identify the degradants observed in the thermal and oxidation degradation sample of Empagliflozin and Linagliptin tablets by using LC-MS and HPLC-PDA instrumental techniques. Thermal and oxidation degradation samples were injected in HPLC-PDA and LC-MS instruments. Mass of the degradants were detected by LC-MS technique, ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market