

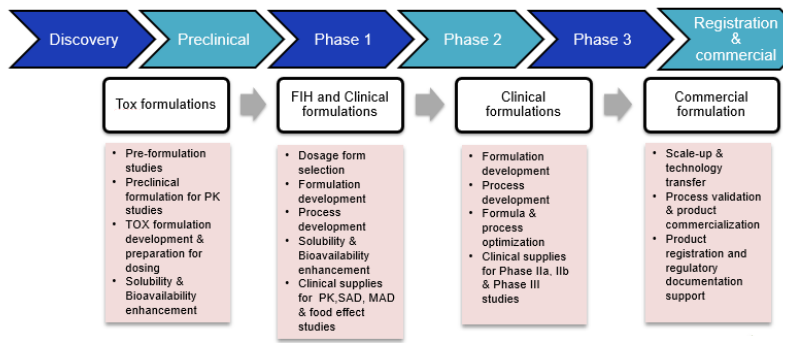
We offer a wide range of formulation services, from preclinical formulation to commercial manufacturing. We have expertise in developing all major dosage forms including orals, parenterals and topicals.
 Speak to our experts
Speak to our experts






Pre-formulation Studies
Pre-formulation studies cover the assessment of a wide range of physico-chemical properties, which are critical to design the formulation and delivery method for your lead candidates. Once the drug properties are studied, our team of experts can help arrive at the right formulation that meets the necessary regulatory requirements.
Early Phase Formulation Development
Our Early-phase formulation development services help solve oral bioavailability challenges encountered at both preclinical and clinical formulation development stages. We can address Low solubility / low oral bioavailability issues through in-depth solubilization and enabling technologies.
Drug Product Development
We offer Drug product development services from GLP-compliant and cGMP environment to meet client requirements. Our formulation development services can help in translating new drug candidates into drug products for your clinical trial needs.
Our services include:
- First in human formulations (Orals, Parenterals and Topicals)
- Clinical formulations (Oral Solids, oral liquids, Topicals and Parenterals- injectables, ophthalmic and otic solutions and nasal solutions)
Clinical Supplies and Commercial Manufacturing
We offer clinical supplies (Phase 1, phase 2, phase 3) and standalone commercial manufacturing services for global markets. Our state- of-the-art formulation manufacturing facilities are approved by major regulatory agencies (USFDA/MFDS/MHRA/ANVISA/TGA).
Clinical and Commercial Packaging Services
Our state of the art cGMP-compliant facilities are equipped with automated packaging machines which provide high efficiency, accuracy and capacity to help speed your product to clinic and commercial stage.
Drug Product Characterization Studies and Testing
Our characterization services for NCEs, generics and first generics help assess the quality characteristics of drug products like physical and chemical properties, which are primary elements to ensure the desired quality, considering the safety and efficacy of the drug product. We help to understand physical, chemical and microbiological properties or characteristics that should be within an appropriate specification to ensure the desired product quality.
We provide customised drug product method development services to support early phase to commercial stage drug product life cycle. Our expert team is well versed with global regulatory requirements for supporting IND/NDA/ANDA filing.
Pharmaceutical Dtability Studies
Our stability testing services offered are as per ICH guidelines which include the study of drug substance and drug product critical quality attributes to support product lifecycle.
CMC and Regulatory Support
We provide CMC compilation support for IND, NDA and ANDA registrations based on customer requirements for global agencies like USFDA, MHRA, MAA, TGA, ANVISA and MFDS. Etc.
Why Aurigene Formulation Services?
Services from early formulation to clinical manufacturing
Integrated API and formulation capabilities
Experience with advanced formulation technologies
Integration with biology services for in vitro and in vivo studies
Global accreditations
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market





