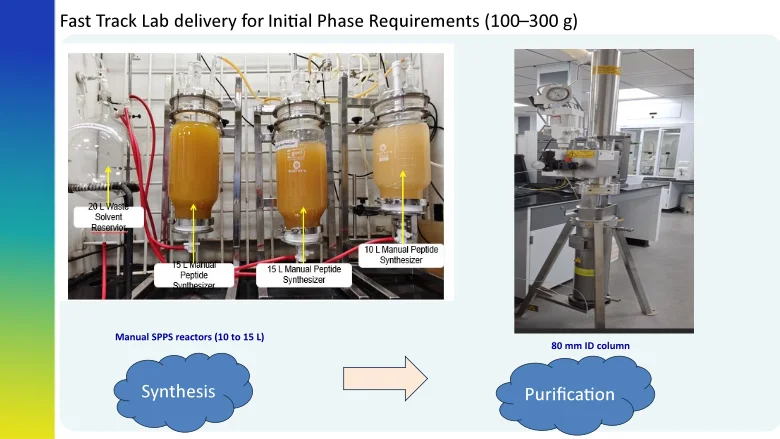
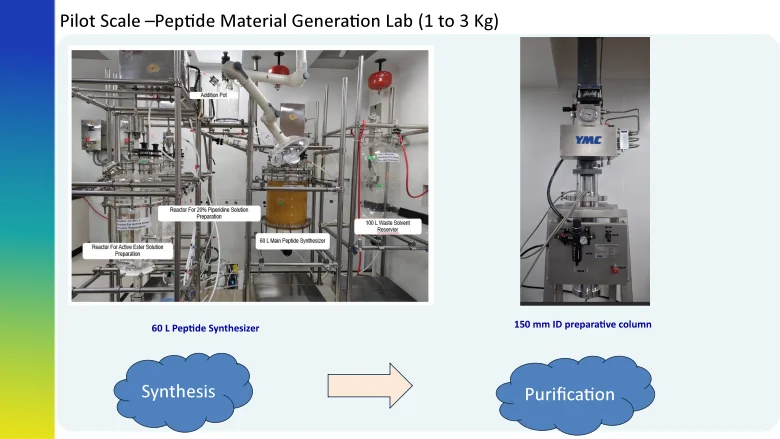
We have a strong track record in process optimization, robust process development, & GMP manufacturing of peptide molecules. Our team is highly experienced in handling various technologies in peptide synthesis− solid-phase, liquid phase, or hybrid approaches− at our R&D centers in Hyderabad and Bangalore. We have dedicated peptide labs to support peptide synthesis, downstream purification, isolation and lyophilization.
As one of the leading CDMOs, we have a state-of-the-art analytical laboratory for impurity characterization and profiling based on spectral methods. Moreover, we have one of the largest facilities to manufacture high-throughput synthesis of a wide variety of standard and complex peptides. Our advanced technology with modern infrastructure enables us to produce up to 3 kg of linear and branched chain peptides at laboratory scale. We provide peptide manufacturing services from our state-of- the-art manufacturing site in Vizag and produce up to 400 kg. The site is inspected by major regulatory bodies such as the US FDA, MHRA, and PMDA.





We can handle a wide range of peptide classes such as:
Backbone modification
- N-alkylated amino acids
- D-amino acids
- N-formyl and acetyl peptides
- Non canonical amino acids
- Peptidomimetics
Cyclization
- Lactam
- Di-sulfide
- Stapled
- Click
- Head to tail
- Side chain to head/tail
- Cyclic/Bicyclic cores
Conjugation / derivatization
- Maleimide derivatization
- PEG’s
- Lipid
- DOTA
- NODAGA
- Carbohydrates
We can manufacture various types of peptide classes with linear peptides up to 40 AA, conjugates of peptides such as small molecules, lipids, carbohydrates, and PEG. In addition, we offer a cost-effective Liquid Phase Peptide Synthesis (LPPS) approach for small peptides and linkers. Our team has deep expertise in process optimization and robust development to ensure reliable and efficient commercial manufacturing.
Advanced Peptide Technologies
Upstream
Solid-Phase Peptide Synthesis (SPPS)
- GMP reactors (SPPS) ranging from 80 to 500 L, enabling production from gram to multi-kilogram scale per batch under cGMP conditions, ensuring flexibility and efficiency
- Automation and high throughput
Liquid-Phase Peptide Synthesis (LPPS)
- Ideal technology for large-scale manufacturing of short peptides
- Hybrid approach
- Fragment-based approach suitable for large-chain peptide synthesis in solution phase, offering a cost-effective second-generation process.
Microwave SPPS (CEM)
- Recommended for high scale requirements (ideal for up to 2 MT scale)
- High purity & yield
- Lesser batch cycle time
Downstream
Purification and Isolation
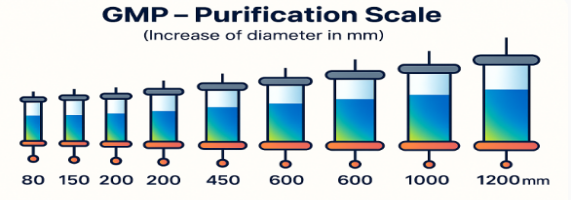
Purification
- RP purification capability with multiple column configurations ranging from 150 mm to >120 cm in diameter.
Isolation
Various isolation techniques:
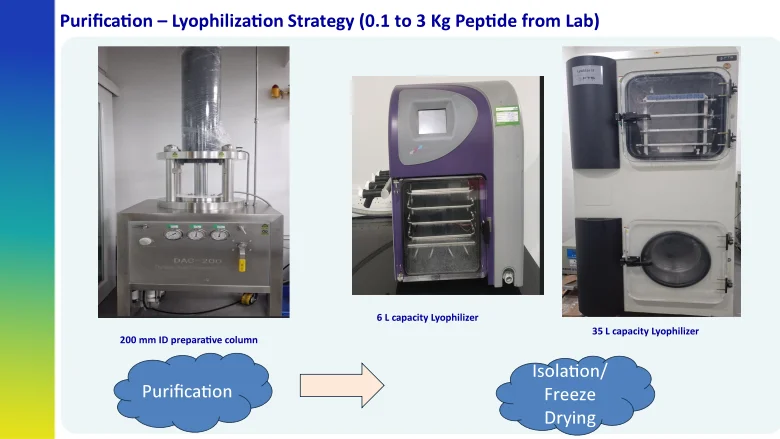
- Lyophilization: Best suited for small- to medium-scale requirements, with multiple lyophilizers available in Class 100K cleanroom areas.
- Precipitation: A highly cost-effective, scalable solution with proven expertise in peptide precipitation, particularly beneficial for large-volume processes.
- Spray-Drying: Suitable for operations at any scale.
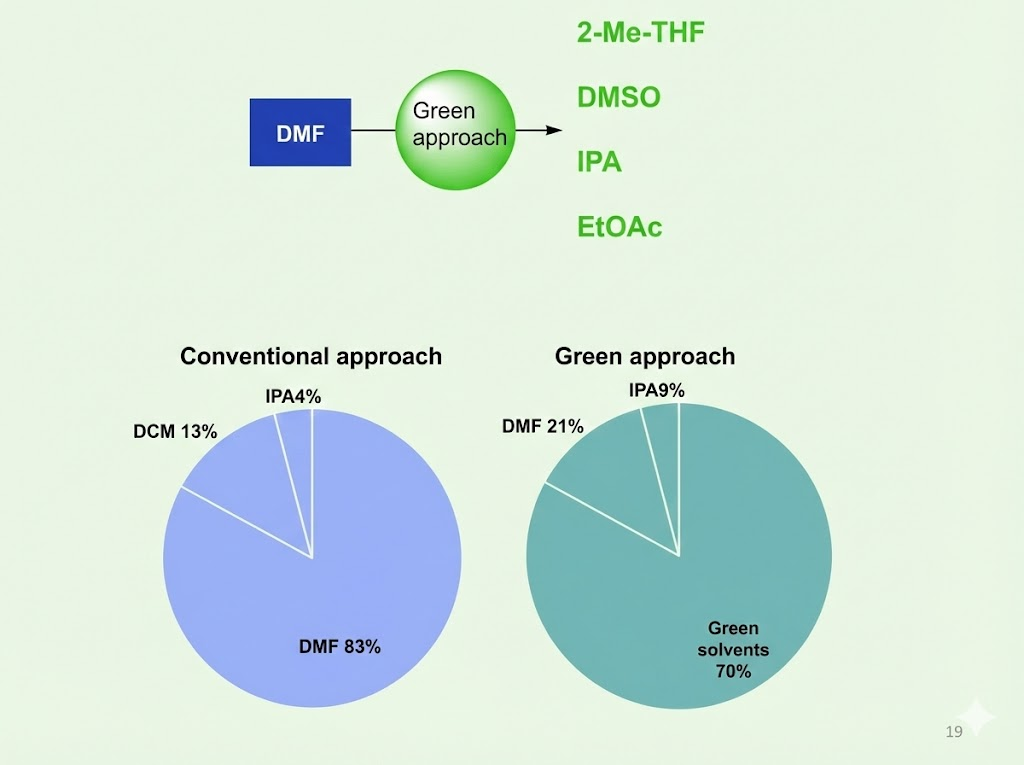
Our green approach for peptide synthesis
- SPPS peptide synthesis uses DMF/NMP. Both DMF and NMP are classified as hazardous and toxic.
- About 70% DMF usage was reduced for 10 mer peptide.
- DIC/OxymaPure were used as suitable coupling reagents along with green solvents.
- 2-MeTHF, DMSO and combination with DMF are appropriate alternative solvents.
- Warming at 40°C for coupling where hindered AA is involved.
- 20% Piperidine in IPA/EtOAc/2-MeTHF affords complete release of DBF.
- IPA / EtOAc can be used in combination washings with 2-MeTHF.
- 2-MeTHF is effective for washings at the end of cycle.
A robust supply chain is essential for successful drug development. Aurigene’s team manages the end-to-end process—from sourcing raw materials and coordinating permits to delivering finished products under fully compliant, traceable conditions.
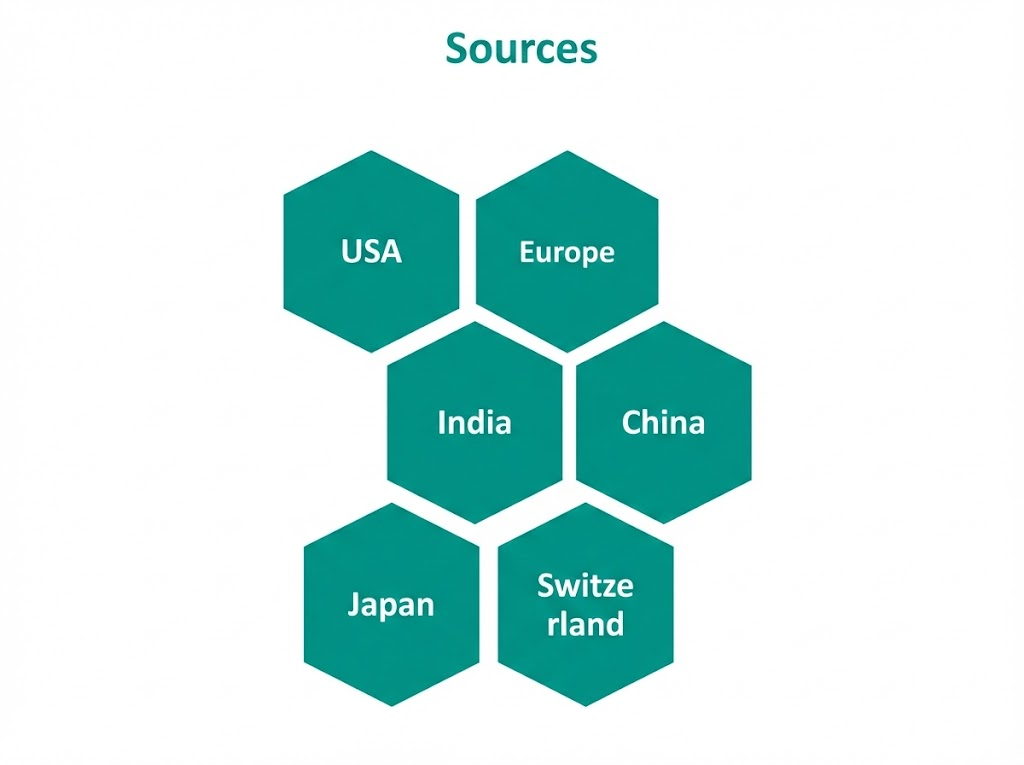
Sourcing philosophy for protected amino acids
- In the upstream process sourcing team arranges the Fmoc amino acids for the synthesis of crude API.
- Down stream process involves the purification of the crude API followed by the isolation.
| Sourcing | Approved sources in India, USA, Europe, China, Switzerland and Japan |
| Certification | BSE/TSE free (Bovine Spongiform Encephalopathy / Transmissible Spongiform Encephalopathy) certified amino acids |
| Synthetic production | Many amino acids are produced synthetically through fermentation processes using bacteria or fungi. This method ensures complete control over the production process, minimizing the risk of contamination from animal-derived materials. |
| Quality control | Regular testing and quality control measures are being monitored. Based on the requirement the site will be audited. |
| Reputation and reliability | We have many approved partners to supply amino acids who have a strong track record. |
| Audits and assessments | Based on the category of the material the vendor's manufacturing site will be audited by our quality team and ensure the sufficient quality. |
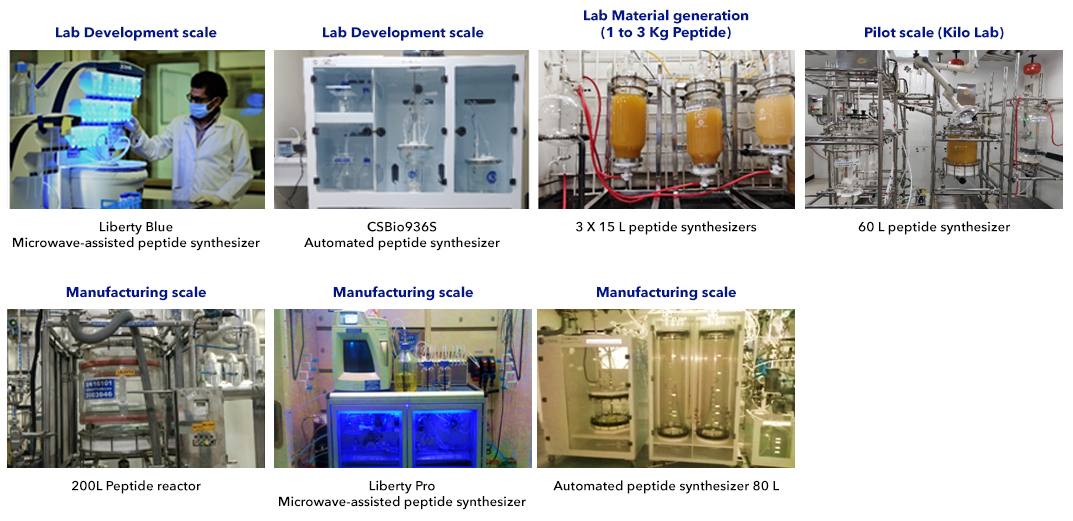
Lab Infrastructure to Support Peptide Development
Synthesizers
- Cost-effective LPPS approach for small peptides/linkers
- Manual reactors ranging from 100 mL to 60 L capacity (SPPS)
- CS Bio 936S: Automated peptide synthesizer suitable for feasibility and optimization studies, pilot studies, and scale-up activities (1 to 5 L).
- CEM automated microwave peptide synthesizer - 2 units
Lab-scale purification
- Multiple preparative reverse phase HPLCs (80,100, and 150 mm).
- MPLC flash system 250 mL/min pump capacity
- Akta explorer ion exchange chromatography
- Aktaprime plus ion exchange chromatography
- Gel permeation chromatography columns, including 150 mm dia., 100 mm dia., and skids for flexible purification.
Isolation techniques
- Crystallization
- Precipitation
- Lyophilization
- Virtis programmable tray of 3 L capacity
- Virtis benchtop of 1.5 L capacity
- 35 L Lyophilizer - Pilot Scale Capacity
- Membrane filtration technology
- Tangential Flow Filtration (TFF) 1 kDa, 10 kDa, and above
Analytical equipment to support peptide development
- LCMS, GC-MS, LC-ToF, LC Orbitrap, MALDI ToF instruments for characterization
- Multi-Angle Light Scattering (MALS) detector for precise molecular weight and conformation analysis in peptide purification workflows.
- Circular Dichroism (CD) analysis for peptides
- 600 MHz nuclear magnetic resonance spectrometry
- Charged Aerosol Detector (CAD) for HPLC
- Amino acid analyzer with post-column derivatization and triple quadrupole LC-MS system for AA sequencing and comprehensive analytical characterization.
Clinical Supply Manufacturing
For your clinical supplies and commercial manufacturing needs, we offer peptide manufacturing from our state-of-the-art sites in Visakhapatnam---inspected by major regulatory bodies including USFDA, MHRA, and PMDA. Facilities feature microwave-assisted technology, Liberty PRO automated synthesizers, and fully automated ion exchange purification systems.
Equipment for Peptide Synthesis
- Automated peptide synthesizer (80, 200, 500 L)
- Liberty pro peptide synthesizer (15 L; 42 units)
- Hastealloy ANFDs of 1m2 capacity for closed loop filtration operations
- Tangential flow filtration
- Separate peptide block (PB – 16) dedicated to peptides & polymers
Peptide Purification and Isolation
- Preparative RP-HPLC (150 to 1200 mm diameter columns)
- Fully automated ion exchange resin systems
- Freeze dryer – Multiple 100 & 200 L capacity (tray)
- Class 100K clean room area with Lyophilizer
Impurity Profiling and Characterization
- LCMS, GC-MS, LC-ToF, LC Orbitrap, MALDI ToF instruments for characterization
- Multi-angle Light Scattering (MALS) detector
- Circular Dichroism (CD) analysis for peptides
- 600 MHz Nuclear Magnetic Resonance (NMR) spectrometer
- Charged Aerosol Detector (CAD) for HPLC
- Amino acid analyzer with post-column derivatization
Equipment:
Peptide Synthesis (SPPS, LPPS & hybrid approaches):
APSL having both:
- Microwave Peptide Synthesizers (CEM)
- Automated Peptide Synthesizer (CS Bio)
Downstream Chemistry

LPPS
Purification & Isolation
Development Scale:

Manufacturing Scale:

Cleanroom

Analytical - Development Site
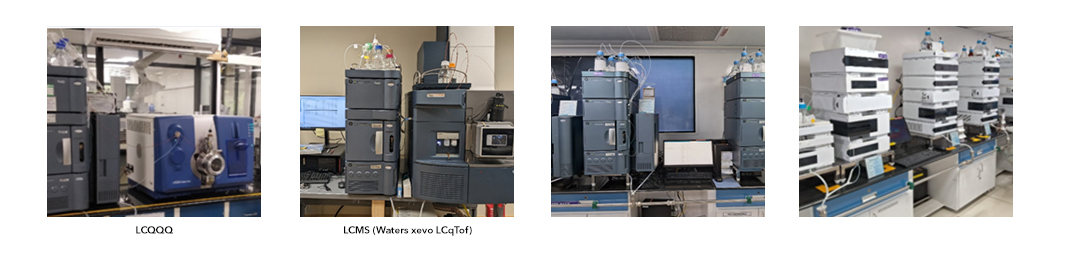
At QC-Manufacturing Plant

Delivery Strategies
Strategy for 100-300 g Delivery of Peptide from Lab
Strategy for 0.5 to 3 Kg Delivery of Peptide from Peptide Material Generation Lab
Purification & Lyophilization Strategy (0.1 to 3 Kg Peptide/Fast Track Delivery)
Why Aurigene Peptide Development and Manufacturing Services?
Aurigene offers advanced solid-phase (SPPS) and liquid-phase synthesis platforms, enabling production from small clinical batches to large-scale commercial supply.
Peptide Synthesis (SPPS, LPPS & Hybrid approaches)
Purification (Prep-C18 & IEX systems)
Isolation (lyophilization, crystallization & precipitation, spray drying)
Analytical studies
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
FEBRUARY 25, 2025
Transforming Drug Discovery with Aurigene.AI
In the early 2000s, developing Sovaldi, a hepatitis C treatment, took over a decade and nearly $2 billion. Similarly, Zolgensma, a gene therapy for spinal muscular atrophy, required 15 years due to its complexity. However, the advent of artificial intelligence (AI) has revolutionized drug discovery. For example, in 2022, Pfizer's PAXLOVID, an oral COVID...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read MoreFamiliarization, process optimization, and cGMP manufacturing and supply of 30.0 kg of a Bioactive Nucleotide (NAD Booster)
Background: A US-based biopharmaceutical company approached Aurigene Pharmaceutical Services for the familiarization, process development, and cGMP manufacturing and supply of 30.0 kg Nucleotide product (NAD booster) for phase-appropriate studies. The synthesis of the desired product involves three linear stages, which starts with reaction of a pentose...
Read MoreIdentification of Degradants of Thermal and Oxidation Stress Studies of Empagliflozin and Linagliptin Tablets by HPLC-PDA and LC-MS Instrumental Techniques
2022
Objective of the manuscript is to identify the degradants observed in the thermal and oxidation degradation sample of Empagliflozin and Linagliptin tablets by using LC-MS and HPLC-PDA instrumental techniques. Thermal and oxidation degradation samples were injected in HPLC-PDA and LC-MS instruments. Mass of the degradants were detected by LC-MS technique, ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
Frequently asked questions
What experience does Aurigene Services have in peptide development and manufacturing services?
Aurigene services provides integrated quality services with an experienced and dynamic team in the drug discovery development and manufacturing domain. As, one of the leading CDMOs, they have state-of-the-art facilities for impurity detection, characterization, and profiling. Leveraging scientific expertise, they manufacture high-quality peptides using solid-phase, solution-phase, and hybrid-phase methods for clinical applications. They have robust process development and optimization for high throughput synthesis of complex peptides, supported by state-of-the-art infrastructure and laboratories. Synthesis is carried out using cost effective LPPS methods and manual reactors, purification using preparative HPLC, gel chromatography, and ion exchange chromatographic methods or resin systems, and isolation using crystallization, precipitation, and lyophilization techniques. Final characterization is performed using circular dichroism and charged aerosol detector, NMR, MASS spectroscopy and multi-angle light scattering detector(MALS), among others.
High-end equipment such as automated peptide synthesizers, Pro Peptide synthesizer, and microwave-assisted peptide synthesizers. Are installed and used for various synthetic operations.
APSL manufactures different kinds of peptides such as linear, branched chain, natural and unnatural conjugated peptide molecules and PEG. They also manufacture peptide derivatives, which are small molecules, carbohydrates, and PEG. They manufacture peptides with disulfide bridges and cyclization achieved through side-by-side or head-to-tail cyclization. Cysteine-rich peptides and lactam- bridge peptides are also extensively synthesized, along with the production of fluorescent tags and dyes.
What are the integrated strategies to accelerate the peptide development in Aurigene Serevices?
Aurigene, one of the leading CDMOs, has one of the largest manufacturing facilities for high-quality, high-throughput synthesis of a variety of complex peptides. The types of peptides that are synthesized range from linear peptides to branched- chain peptides, to peptide derivatives, peptides with disulfide bridges, cysteine-rich peptides, lactam-bridge peptides, stapled, peptides, fluorescent-tagged peptides and dyes, and conjugated peptides. Aurigene brings deep experience and expertise in process development, with strong capabilities in process optimization and robust commercial-scale manufacturing. Aurigene has been manufacturing highly reliable and robust peptides using solid-phase, solution-phase, and hybrid-phase synthesis in a cost-effective manner. The company also offers GMP manufacturing services to meet both clinical and commercial requirements.
With the state-of-the-art facilities, advanced laboratory infrastructure, and robust equipment maintenance practices, Auriegene provides peptide development services in a wide range. The laboratories are equipped with synthesizers with manual reactors, automated peptide synthesizers, and automated microwave-assisted peptide synthesizers. Purification is performed using preparative chromatographic techniques, gel permeation, and ion exchange chromatographic techniques. Impurity characterization and profiling are performed using various spectral methods. Isolation techniques include crystallization, precipitation,lyophilization and membrane filtration technologies. A wide range of analytical methods, such as LC-MS, GC-MS, and other advanced techniques to support peptide characterization.
How do we ensure quality control in peptide development and manufacturing process?
Quality control is a critical function applied to analytical testing and characterization of raw materials and finished products, forming an integral part of the manufacturing process. It maintains the documented data’s corresponding to raw materials and products to ensure compliance with pre-established specifications. All analytical methods are developed and validated in accordance with Good Manufacturing Practice (GMP) guidelines. During peptide development, assessing structural dynamics and purity is critical. Analytical testing methods play a role in analyzing, identifying, and characterizing impurities that can later may recur and interfere with the development process, thereby compromising the drug product profile.
There should be process optimization and robust method development for commercial manufacturing. Analytical data can verify and validate impurity characterization and profiling with the help of spectroscopic methods to develop high-quality peptides. Quality control is used to validate a variety of peptides and peptide quality control and assurance are managed at every step during the development and production to meet regulatory requirements. It ensures that the analytical results of the peptide analysis fall within acceptable limits of precision and accuracy, so that the quality and purity of the synthesized peptides are not compromised. Purity assessment and analysis are performed through analytical reverse phase HPLC and characterization through various techniques of mass spectroscopy and UV detection. The safety assessment is a critical factor in understanding the structure, physicochemical properties, stability, and impurity profile of the concerned peptide molecule.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market