
The Investigational New Drug (IND) application and New Drug Application (NDA) are critical steps towards registration and transportation/distribution/marketing of a drug product across geographical regions. They are also critical towards delivering safe and effective drugs to patients across the globe. Any delays/setbacks can cost the drug developer in terms of time and resources, making it essential to understand the intricacies of both processes and accelerate the registration process. The drug regulatory agencies such as the FDA require that an IND/NDA application must contain information on non-clinical Pharmacology and Toxicology studies apart from manufacturing information, clinical protocols and investigator information.
Few of the critical factors that plays a vital role in drug development are identifying the safety liabilities of the new drug candidate at the early phase of discovery itself and generating convincing pre-clinical safety toxicology data for the right drug candidate. The generated preclinical data (exploratory and definitive) will help the regulator to permit an assessment as to whether the product is reasonably safe for initial testing in humans and further approvals.
Pre-clinical toxicology evaluation strategies, specific but not limited to identifying toxicity mechanisms, selection of right species for toxicity testing, their relevance towards translatability of the findings to humans, identifying target organs, safety markers etc., should be in place to mitigating risks and identifying side effects at the earliest possible. These early findings might help to assess the degree of toxicity with convincing clarity, which will help the clinicians and pharmacologists to calculate safety margins and aid in a significant manner to have a stop-go decision at a very early stage of drug development.
Attrition due to safety concerns occurs at all stages of drug development and constitutes a large fraction of the failure rate of new chemical entities, non-clinical toxicology studies being one of the factors. Avoidance of toxicity-related attrition at the later stage of drug development is one of the critical factors that should be considered during the early drug development phase itself. The goal of non-clinical exploratory toxicity studies in the discovery stage is to integrate information from a variety of liabilities and assess if a new compound has the profile to become a drug.
Exploratory toxicology screening strategies will help to accelerate the drug development process enabling early identification of hazards and risk mitigation. These strategies include in silico predictions, molecular and phenotypic screening, tailor-made in vitro cellular and biochemical screening assays and, in vivo exploratory toxicity studies covering secondary pharmacology and pathology models, which are designed and carried out as the identified drug candidate progresses. This strategy not only reduces attrition, but also increases the likelihood of success of the program so that ensuing non-clinical regulatory toxicology programs and clinical trials can be successfully executed.
Another step to successful drug discovery is to combine available toxicity data with other drug specific features covering but not limited to absorption, distribution, metabolism and excretion (ADME) and physicochemical properties of the drug candidate. Recent advancement in these areas include the use of advanced micro-physiological systems (organ-on-chip) and imaging techniques, stem cells, 3D tissue models etc. Few of the current assays and methods used in the exploratory toxicology space are high throughput molecular/cellular imaging, toxicokinetic vs toxicodynamic mathematical models that can assess safety risk and bioinformatic analyses to identify mechanisms. Few assays/ methods under development and evaluation are in vivo imaging techniques targeting to observe live physiological functions, organ-on-chip to simulate human biology of multiple organs, targeting specific target gene deletions/mutations using CRISPR, human cells derived from stem cells (iPSCs) to profile drug candidates, integration of classical toxicity assessment methods with respective models to identify molecular and functional changes, tissue specific microRNA profiles as biomarkers for specific toxic effects, evaluation of mechanism of toxicity using metabolomics and mass spectrometry imaging enabling detection of drugs, metabolites etc., from tissue sections relating them to pathological changes.
Toxicity Screening Approaches – Current and Under Development/Future
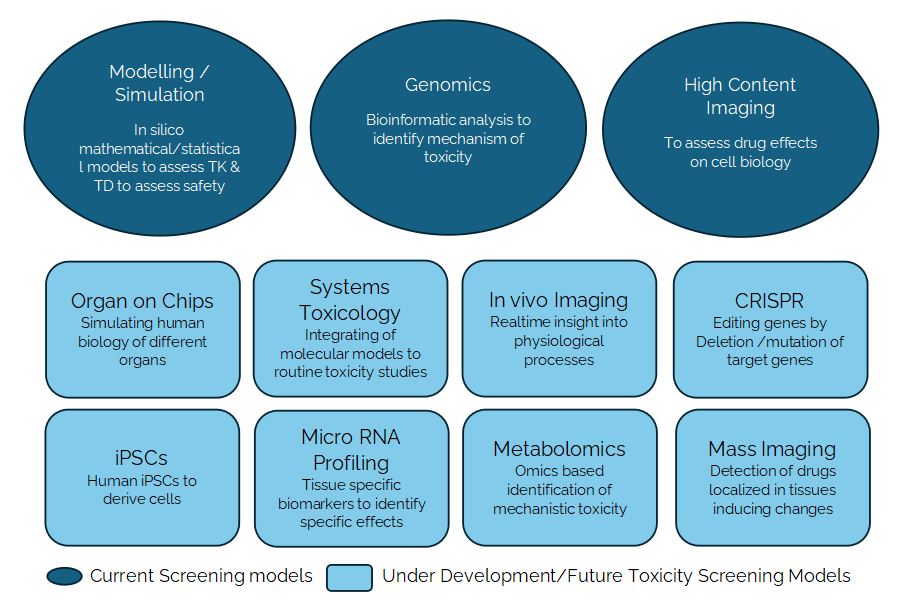
In brief, exploratory toxicity studies carried out at the early stage of drug development help in the prediction of potential adverse effects prior to conduct of non-clinical definitive regulatory toxicity studies and clinical trials and to identify the mechanism through which toxicity is elicited. These studies will aid in the identification of safe drug candidates to progress further and to eliminate those that express severe adverse effects at a very early stage of drug development.
With the help of data available from the exploratory toxicity programs, pre-clinical toxicology studies conducted in compliance with Good Laboratory Practice (GLP) regulations can be designed appropriately through the selection of right species and the selection of the right target parameters to be evaluated. This would result in the generation of meaningful scientific data which could be provided to the pharmacologists and clinicians to aid in the selection of human doses and valuable information towards identifying adverse effects during the clinical trials. GLP has contributed significantly to augmenting the quality of drug development around the world for decades by establishing criteria and defining standards ensuring the quality and integrity of nonclinical safety data submitted to regulatory bodies.
Toxicology Testing Paradigm
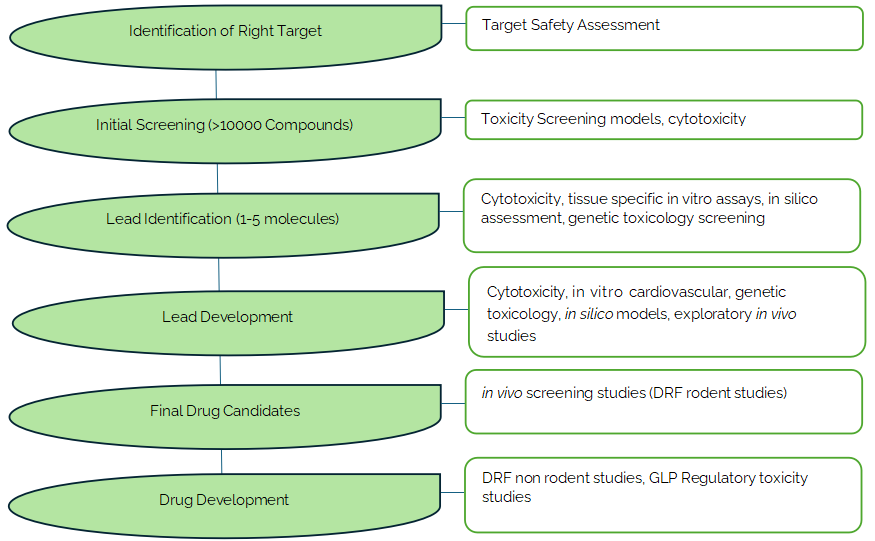
In the US, early communications between drug developers and new drug review divisions is possible with CDER's Pre-Investigational New Drug Application (IND) Consultation Program which further helps to generate data that is necessary to warrant IND submission. This will save a lot of time and energy as the roadmap on the type of non-clinical safety toxicology studies those are required for a particular molecule on a given therapeutic target is defined clearly and agreed upon by the regulator and the drug developer before initiating the definitive toxicology program.
Selection of the right exploratory toxicology screening strategy, careful designing and conduct of non-clinical GLP toxicology studies based on the data derived from exploratory toxicology studies and early communications/discussions with drug regulatory bodies will aid in developing a robust drug development program which would help to accelerate the process of identification of the right drug candidate, its non-clinical and clinical development thereby accelerating IND/NDA approvals.
About Author:

Dr. Navin comes with 26+ years of work experience in basic and regulatory research front, having experience in the field of nonclinical research towards hazard and risk assessment for pharmaceuticals, vaccines, agrochemicals and biosimilars.
A core investigative member for evaluating and presenting safety data to DBT/ICMR/DCGI/FDA/EMA/WHO committees for various therapeutic research programs and till date succeeded with a number of products into clinical trials and towards global market approvals.
Notable achievement in leading the pre-clinical toxicology team who developed Enmetazobactam, a novel betalactamase inhibitor, which is currently being marketed in Hong Kong and is approved in Europe for marketing. A member of various research project committees has several publications to his credit. Well acquainted with OECD-GLP and AAALAC compliance driven process. Experienced in conducting due diligence inspections representing multinational pharmaceutical companies and performed leading role in collaborative research programmes with other R&D setups.
Experienced in developing pharmacology models and mechanism based toxicity experiments, chemical carcinogenesis, toxicokinetics and ADME liabilities and generating non-clinical dossiers. Was a primary contributor to the planning and construction of the Pre-clinical Facility at Orchid Chemicals & Pharmaceuticals Ltd., which eventually became the first Indian GLP accredited Pharmaceutical Laboratory in India.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market




Add new comment