

The 2023 Nobel prize in Physiology and Medicine was awarded to Prof. Katalin Karikó and Prof. Drew Weismann for their ‘contribution and discoveries concerning nucleoside base modifications that enabled the development of effective mRNA vaccines against COVID-19’.
The vaccine for COVID-19 has taken record time and one of the fastest made vaccine in the history. It took only ten months, a record time to make it available on the market.
At that time common people were hesitant about the newly known vaccine technology, called as messenger RNA (mRNA) technology. Some of us were in a great dilemma whether it would work properly as it was developed unprecedently so fast. The name mRNA technology was very new to us for obvious reasons all the while. Unfortunately, we were unaware that hundreds of scientists were working on the development of this field more than three decades before the pandemic started. Most probably this could be the very basic reason to become apprehensive about it.
This article will focus on the brief history of mRNA technology and the future applications in this discourse.
What is the conventional method of vaccine synthesis?
In 1951 Nobel prize in Physiology and Medicine was awarded to Max Theiler for the discovery of yellow fever vaccine. It was a decade long research and innovation. Jonas Edward Salk, a well-respected American virologist and medical researcher, discovered the first safe Polio vaccine in New York in 1955. It has big impact on the vast populus country like India to fight against the dangerous effect of Polio. It took 10 years of hard work and understanding. The record breaking four years was taken for the discovery of the Mumps vaccine by Morris Hillman and his team.
Usual vaccine was made by either administering of a dead or weak virus otherwise a portion of the virus body parts in the body. The foreign elements are easily identified by our immune system and trigger the synthesis of corresponding antagonist proteins. Therefore, comprehensive virus culture following all the protocols is required to develop a vaccine. Willing volunteers for clinical trials are an integral requirement. In this process, the virus culture, required trials and vaccine preparation need huge time and regulatory precautions. Moreover, conventional vaccine approaches may not be applicable to non- infectious diseases, such as cancer [1]. Therefore, the rapid development of more potent and versatile vaccine platforms is on high demand. We can take Covid-19 situation as a vivid example in this regard.
What is mRNA?
mRNA terms refer to the messenger ribonucleic acid, an essential part of our cell. It is ribonucleic acid unit that has the responsibility to transfer the information from our gene or DNA to ribosome to generate a particular protein. As a carrier mRNA plays the role to transfer the information from RNA to ribosome. After transferring the information, mRNA gets demolished in a natural way. The mRNA never gets back to the nucleolus or DNA.
Therefore, it’s a very useful tool to generate any protein (of our choice) by incorporating a transcribed mRNA in cell. Thus, this newly formed protein will trigger the immune system and the cell will start action accordingly against it. You can see that, in this way we can avoid the virus culture and all the required regulatory affairs etc. However, the real challenges were the stability of mRNA and inception of mRNA inside a cell.
Brief about mRNA research:
The mRNA was discovered in 1961, but it took almost six decades to get the first FDA approved vaccine. The synthesis of mRNA was not achieved till 1984. During this period scientists were isolating mRNA from cells and using it for their research. In 1984 a group of scientists (Krieg, Douglas Melton, Tom Maniatis and Michael Green et al) of Harvard University discovered the RNA-synthesis enzyme (taken from a virus). Owing to “unbelievable instability” it was not feasible to synthesize RNA in laboratory since then.
In 1987 Robert Malone described a new technique to incorporate mRNA into a cell. He mixed strands of mRNA with droplets of fat and successfully transfer it into the human cells, which began producing proteins from it. Based on the evidence that incorporated mRNA can produce protein of our choice inside the cell, in 1988 (January) Malone realised and envisaged that mRNA could be treat as a drug in future. The following years Malone successfully shuttle the mRNA into the frog embryo. At that time, it was remarkable to incorporate mRNA into a cell using fatty acid. Robert Malone’s approach was based on this discovery where he used cationic liposome to channelize mRNA inside the cell.
In 1991, Vical along with Merck started the initiative for mRNA technology in mice with the aim of creating an influenza vaccine but drop the idea keeping eyes on the cost and feasibility of manufacturing. In 1993 the first mRNA vaccine was evaluated in mice for influenza virus.
Regardless of the various limitations viz. mRNA instability, high innate immunogenicity, translational efficiency, inefficient in vivo delivery, and above all the stringent funding, some of the scientists didn’t drop the idea. Prof. Kariko was one of them who struggled in the face of all odds.
After her postdoctoral study Dr. Kariko joined Pennsylvania State University in 1989. Despite her high spirit to work on the field of mRNA-based gene therapy she failed to impress the funding agencies for the research grant. During that period neither the researchers, experts nor the funding agencies had confidence on this research topic for various reason. It was a tough time for her indeed. Dr. Kariko met the immunologist Dr. Weismann in a photocopy machine in 1997. Their short interaction last as a long and fruitful collaboration in the field of mRNA research.
Journey of mRNA vaccine:
In the late 1990’s the Merix Bioscience tried to apply the mRNA technology in the field of oncology. However, the late-stage large trial was not seeing the light of success. But this research work gave the insight and inspiration to both Igmar Hoerr, at CureVac and Uğur Şahin, at BioNtech to start the mRNA strategy for their next venture.
The advantage of mRNA base protein generation in our body is – no extra effort is required for the preparation and inception of desired protein into the cell. Keeping eyes on this particular aim Kariko and Weismann were trying to prepare the mRNA that could serve the purpose without disturbing the cell equilibrium. However, most of the cases they observed the inflammatory response by the cell by in vitro transcribed mRNA. The living system treated the incorporated mRNA as a foreign element and activate mammalian innate immune system. Now the question is how to avoid “toll like” receptors (TLRs), responsible to trigger the immune system?
Initial doubt was the structure of the transcribed mRNA that was synthesised in laboratory. In due course they observed that the tRNA has comparatively less inflammation effect. As they studied the structure of tRNA they found that, out of four base pairs (Adenine, Guanine, Uridine and Cytosine) the structure of the uridine is a modified one in tRNA. This observation acknowledge the idea of modification of mRNA by using alternate uridine e.g., pseudo uridine etc.
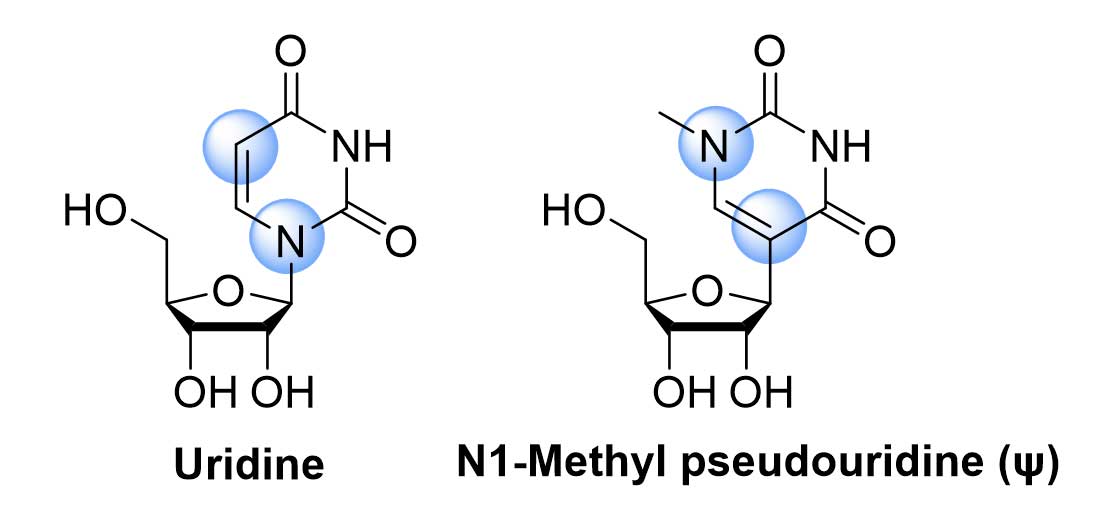
Figure-1:Uridine and N1-Methyl pseudouridine
Among the various possibilities of the modification of uridine the N1-methyl-pseudouridine (ψ) gave the desired result. Thus, the modified mRNA, consists of N1-methyl-pseudouridine, diminish the activity of innate immune sensor, improve the translational capacity, and increase the protection of mRNA from the nuclease (Figure-1). This revolutionary article published in 2005, was one of the milestones of this mRNA vaccine technology. The two laureates showed that, the base modified mRNA also reduced the activation of PKR (protein kinase R) i.e., increased protein production was observed. Along with this, degradation of base modified mRNA get reduced significantly (recognition by 2’5’ oligoadenylate synthetase (OAS) and degradation by the OAS-induced Rnase L enzyme).
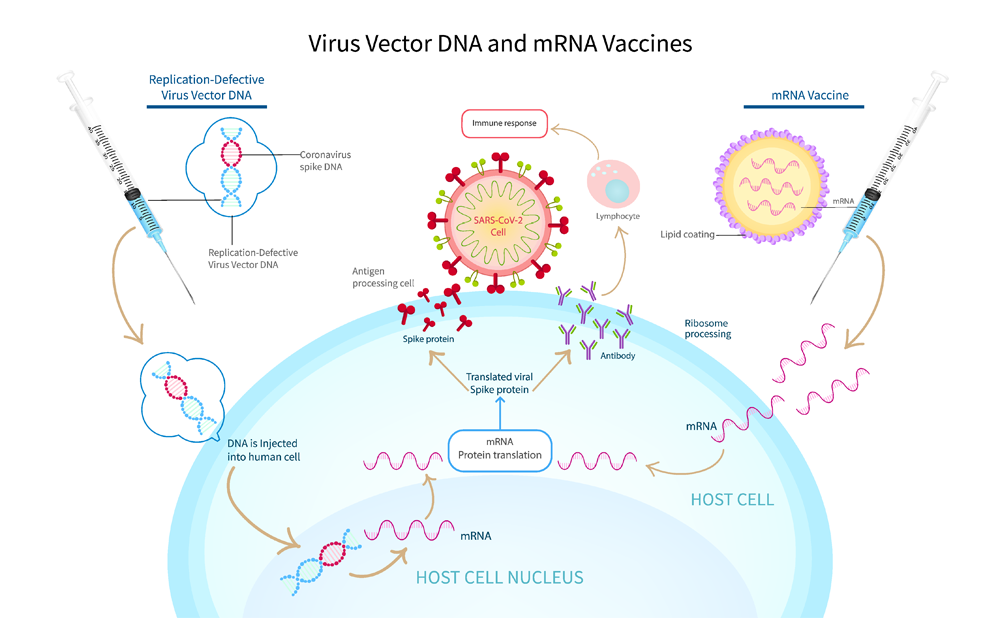
Figure-2:Virus vector DNA vs mRNA vaccine
Does the modification of mRNA require for the vaccine?
The scientist revealed the structure of corona virus soon after the pandemic started using cryoelectronic microscopy. The spike protein encoded mRNA with modified (N1-methyl-pseudouridine) and unmodified uridine are the two approaches for the vaccine synthesis. The modified mRNA was used by both Pfizer-BioNTech and ModernaTherapeutics presented efficacies higher than 90% on clinical trials. Whereas Curevac mRNA vaccine (CVnCoV), consisted of unmodified mRNA, furnished efficacy only 48% in clinical trial (doi: 10.3389/fcell.2021.789427). This sharp contrast in efficacy could be attributed to the importance of modified mRNA technology over the unmodified counterpart.
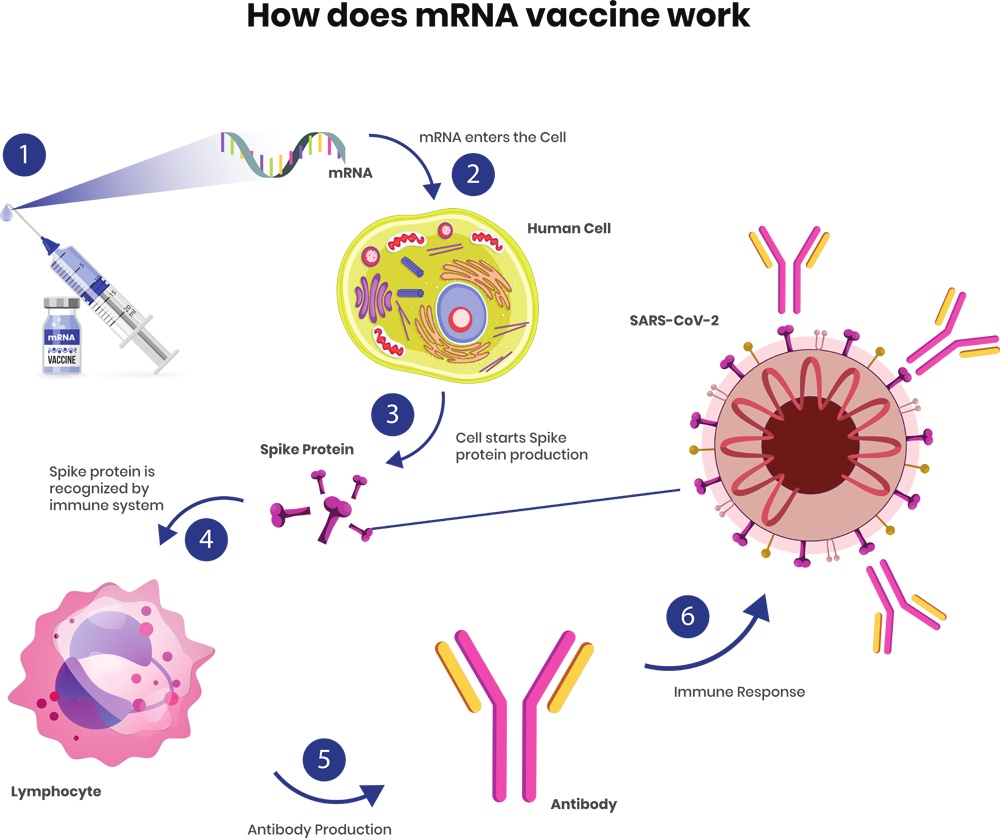
Figure-3:How does mRNA vaccine work
There is no magic but hard work:
This brief discussion is only the tip of the monumental basic research and optimization on mRNA research performed by the hundreds of scientists in last few decades. Truly there is no magic but hard work is the deciding factor to get the SARS-COV-2 vaccine withing a year. However, the advantages of mRNA technology are still a burning question in our mind.
First the safety: as mRNA is a non-infectious, non-integrating platform, and degraded by regular cellular process, it practically carries no risk of infection or insertional mutagenesis. It’s in-vivo half-life can be controlled by suitable modification.
Second the efficacy: the stability of modified mRNA, efficient in vivo delivery method and minimum genetic vector increased the efficacy of mRNA vaccine.
Third the production: mRNA vaccine production speed is high, inexpensive, and less complex scalability issue are the main advantages in production.
Future mRNA vaccine(s) and Conclusion:
Diverse mRNA vaccine research is going on around the globe. Companies like National Institute of Allergy and Infectious Disease (NIAID), Sanofi, Pfizer and Moderna etc. are actively working on the mRNA technology-based influenza vaccines. Infection of Zika virus to the pregnant women caused stillbirth, miscarriage, and birth defects in an unborn baby. Moderna is involve in the Zika virous vaccine as there are no vaccine available till date. Despite of the tremendous difficulties researchers participate in the development of HIV and cancer vaccine. Hope in near future we will witness more mRNA-based successful vaccine available in the market.
References:
- 1. Nature Review, 2018, 17, 261
- 2. Immunity, 2005, 23, 165 (doi: 10.1016/j.immuni.2005.06.008)
- 3. Molecular Therapy, 2008, 16, 11, 1833
- 4. Nucleic Acids Research, 2010, 38, 17, 5884
- 5. Nature, 2021, 597, 318
- 6. Front. Cell Dev. Biol. 2021, vol 9 (doi: 10.3389/fcell.2021.789427)
- 7. https://www.nobelprize.org/prizes/medicine/2023/summary/
About Author:
Amalesh Roy is a Specialist in the Process Research Department (PRD) of Aurigene Pharmaceutical Services Pvt. Ltd., Bangalore. He pursued doctoral work at the Indian Association for the Cultivation of Science (IACS), Kolkata, in the field of synthesis of biologically active natural products. Later he worked at IIEST, Shibpur (UGC-Dr. D S Kothari fellowship) and Max-Planck Institute for Coal Research, Germany as a Postdoctoral research fellow. He has eight years of experience working as a process chemist in the leading pharma and agrochemical industries viz. Sun Pharmaceutical Industries Ltd. (Gurugram) and Syngenta Biosciences Pvt. Ltd. (Goa). He has been engaged as a science communicator with bigyan.org.in, a leading Bengali webzine, for ten years to bridge the gap between science research and popular understanding of science.
Latest Posts

Good practices in non-clinical toxicology assessment to accelerate IND and NDA Submissions
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market





Add new comment