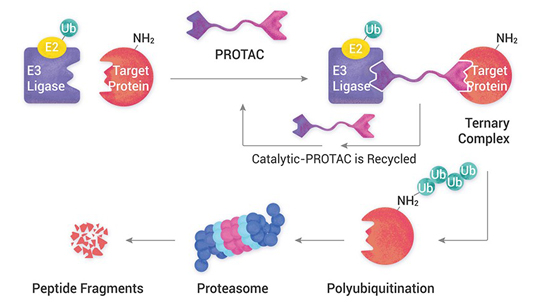
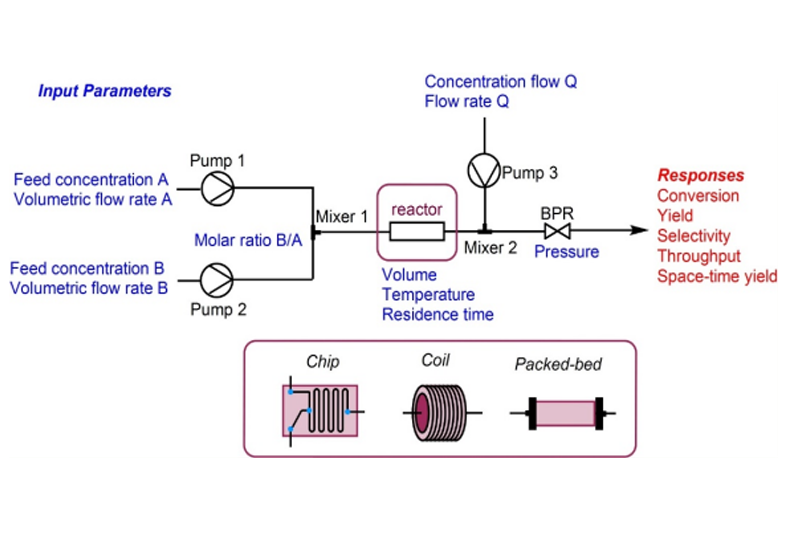
Technology transfer of API from lab to plant is a very critical process for successful API delivery to the client. Diligent planning and execution are required right from selecting plant and equipment, obtaining manufacturing slots, reviewing product development and ensuring the right documentation is delivered on time.
At APSL, we have a cross-functional team of analysts, chemists, engineers, and quality specialists who lead the process of technology transfer. We have extensive experience of scaling up 500+ projects and 1500+ process steps. Our technology transfer procedure from the laboratory to the respective manufacturing facility includes:
Process safety clearance & confirmation of equipment train
Analytical method transfers for raw materials, intermediates, in-process tests, and final compound
Sharing of process understanding, preparation, and approval of Batch Production Records (BPR)



The following are the steps followed in technology transfer:
Process safety clearance and confirmation of equipment train
At APSL, we believe in a safe and sustainable manufacturing approach. Controlling chemical reactions and associated hazards is an important aspect of chemical manufacturing. Almost 30% of incidents are caused by chemistry issues due to inadequate study during the developmental phase. Hence, we strive to mitigate hazards and associated risk in scale up by practicing process safety to a great extent.
At APSL, there is a very structured methodology for performing process safety. The process includes:
- Literature data and calculations
- Basic screening tests
- Isothermal calorimetry to find out heat of reaction for process
- Adiabatic calorimetry to examine the runaway potential of reactions and compounds and specific measurements like vent sizing calculations
- Generating Hazard Evaluation Report (HER) for scale up assurance
- Based on the safety evaluation, appropriate engineering controls will be adopted prior to batch processing and they will be captured in the Process Hazards Analysis (PHA) and Process Safety Information (PSI) report.
- Pre-Startup Safety Review (PSSR) will be carried out prior to the start of the batch as a checklist on safety to ensure whether all the safety recommendations are being implemented in the specific manufacturing unit or not. Only after that, batch start-up clearance is given on the specific equipment train.
Analytical method transfer for raw materials, intermediates, in-process tests and final compound
We have internal Standard Operating Procedures (SOP) which govern the analytical method transfer process. Transfers shall be initiated with pre-approved protocols, followed by batch analysis. SOP guides will fix the acceptance criteria for individual tests to conclude the completion of analytical method transfer.
Decision on slots at the plant
After completion of process optimization, we propose the technology transfer date in consultation with the technology transfer team and technology absorption team.
Stage gate meetings
PR&D, AR&D, and Process Engineering shall submit the development reports to QA three working days before the proposed technology transfer date. QA shall conduct a gate meeting at least two days before the technology transfer date to review the product development of API using a gate checklist (based on the scope of the project, regulatory submissions/GMP requirements, etc.) Timelines shall be provided for the pending action items in the gate checklist. Head QA or designee shall decide on the clearance for the technology transfer depending on the pending action items.
Technology transfer
Technology transfer shall be conducted based on an optimization study or familiarization report. QA shall hand over all documents along with the technology transfer checklist to the concerned plant QA.
The data generated (document transfer) at the development center will be transferred to the manufacturing facility which would include:
- Raw material list along with specifications and method of analysis
- Optimization report / finalized process for the manufacturing
- Holding study data of reaction mass, wet/dry intermediates
- Recovery/reuse of solvent (as applicable)
- Reprocessing / rework procedures (as applicable)
- Details of analytical methods for in-process controls, intermediates, and final compound
- Reference standards
- Process flow diagram
- Equipment justification reports
- Details of process safety evaluation and the recommendations
Based on the technology transfer document batch production record to be generated prior to manufacturing start up
Approval of BPR and start of manufacture
After completion of the technology transfer, the required Batch Production Records (BPR) will be generated based on the details provided by the technical team and further will be reviewed and approved by the Quality Assurance (QA) team. Post-completion of the BPR approvals, manufacturing will follow.
We will share the BPR (before it is reviewed and approved by the internal QA team) with the customer team for information purposes only. However, if there are any specific comments from the customer, the BPRs will be further refined as required.
Why Aurigene Technology Transfer Services?
Experience in scale up of 500+ projects or 1500+ stages
Availability of fast accessible non-GMP pilot plant for risk mitigation
Multifunctional technology transfer and absorption core team
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
FEBRUARY 25, 2025
Transforming Drug Discovery with Aurigene.AI
In the early 2000s, developing Sovaldi, a hepatitis C treatment, took over a decade and nearly $2 billion. Similarly, Zolgensma, a gene therapy for spinal muscular atrophy, required 15 years due to its complexity. However, the advent of artificial intelligence (AI) has revolutionized drug discovery. For example, in 2022, Pfizer's PAXLOVID, an oral COVID...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read MoreFamiliarization, process optimization, and cGMP manufacturing and supply of 30.0 kg of a Bioactive Nucleotide (NAD Booster)
Background: A US-based biopharmaceutical company approached Aurigene Pharmaceutical Services for the familiarization, process development, and cGMP manufacturing and supply of 30.0 kg Nucleotide product (NAD booster) for phase-appropriate studies. The synthesis of the desired product involves three linear stages, which starts with reaction of a pentose...
Read MoreIdentification of Degradants of Thermal and Oxidation Stress Studies of Empagliflozin and Linagliptin Tablets by HPLC-PDA and LC-MS Instrumental Techniques
2022
Objective of the manuscript is to identify the degradants observed in the thermal and oxidation degradation sample of Empagliflozin and Linagliptin tablets by using LC-MS and HPLC-PDA instrumental techniques. Thermal and oxidation degradation samples were injected in HPLC-PDA and LC-MS instruments. Mass of the degradants were detected by LC-MS technique, ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market