We operate our Discovery Biology services from our R and D centers in Hyderabad and Bangalore. The facilities are equipped to perform standalone services and integrated services across In vitro biology, DMPK, In vivo pharmacology, Microbiology and Toxicology services.
In vitro Biology
In vitro lab at Aurigene is equipped to perform standard and highly specialized biochemical and cell-based assays across different disease areas, multiple targets and a broad range of drug classes. In addition to the regular instruments like plate reader, biosafety cabinets, we have high end instruments for quantification of drug to protein interaction.
Videos
DMPK
DMPK lab infrastructure covers a lab area of 10,000 sq.ft. with in vitro ADME and tissue culture labs. We operate GLP as well as Non-GLP bio analytical labs with high-end instruments including LC-MS/MS, Sciex API, Waters Xevo TQS, HPLCs/UFLCs with UV, PDA and fluorescence detectors. The in vivo and PK studies are conducted in AAALAC accredited animal facilities. In addition to the above, we also have a compound management and data automation system set up for DMPK.
In vivo Pharmacology
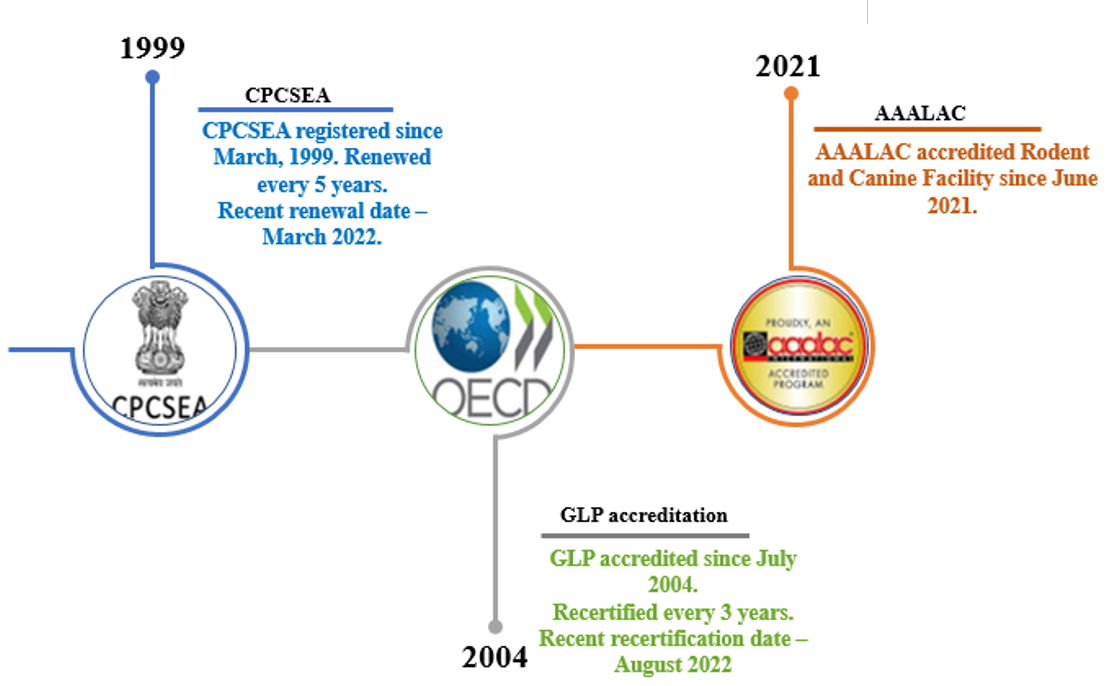
We have an AAALAC & CPCSEA accredited animal facility through which we can handle 600 rodents at any given time. We have more than 50 in vivo efficacy validated models available for testing. We provide PK/ PD correlation studies, biomarkers establishment studies across various targets and have advanced equipment for testing of compounds at early-stage drug discovery including anesthesia machine, electronic vonfrey, randall-sielto, capacitance meter, plethismomteter. We have completed 1000+ efficacy studies including submissions to the US FDA and DCGI.
Microbiology and testing
Our microbiology laboratory is biosafety Level 2 compliant, and it has been audited by US FDA in 2019 with Zero 483 observations. The labs are well equipped with all required instruments like biosafety cabinets, shaking incubator, carbon dioxide incubator, spectrophotometer, microscope and automatic microbial colony counter. We have a media laboratory where all types of microbiological media are prepared in-house using dehydrated powders.
Toxicology
We offer broad range of toxicology services from our GLP certified toxicology lab. We have an experience of conducting more than 9000 toxicity studies during the past 20 years. All toxicology studies are conducted in accordance with national and international test guidelines and in compliance with OECD principles of Good Laboratory Practice (GLP). The animal toxicity studies include short and long term non-GLP exploratory and GLP IND toxicity studies. The tests are carried out in rats and mice, and are maintained individually in ventilated cages. Our animal facility is AAALAC accredited. Our expert team of scientists are proficient in conducting assays such as bacterial reverse mutation test, in vivo and in vitro micronucleus test, in vivo and in vitro chromosomal aberration test. Clinical pathology laboratory is well equipped with fully automated analyzers for hematology, coagulation, clinical chemistry, and urine analysis. Histopathology laboratory is well equipped with state-of-the-art instruments to prepare good quality slides for various organs and tissues collected from experimental animals. These histopathology slides are examined microscopically by expert veterinary pathologists for safety evaluation of test articles. Apart, from standard toxicity assays, we also perform customized toxicity studies as proposed by the sponsors.
Animal Facility/Vivarium
To support our numerous discovery biology preclinical services, we have established and validated animal models for efficacy, pharmacokinetics and toxicology services. The vivarium is AAALAC accredited since June 2021 in appreciation of its animal welfare policies and for the care and use of laboratory animals We follow experimental protocols approved by Institutional Animal Ethics Committee (IAEC) / registered with Committee for Control and Supervision of Experiments on Animals (CPCSEA). The toxicology lab was established in the year 2002 and has been a OECD-GLP certified testing facility since 2003. Our vivarium has pollution board registered bio-waste disposable management systems and our institution has registered Institutional Biosafety committee ( IBSC) under department of Biotechnology, India .
Monitoring Systems
There are numerous monitoring systems available to help you understand experimental and other parameters.
- Rodents are procured from subsidiaries of Taconic and Charles River breeding facilities in Hyderabad. Select rodents and beagle dogs (Charles River and Harlan Laboratories) are bred in-house.
- The vivarium has restricted access control and CCTV monitoring system.
- Veterinary examination is regularly done, feed store area and four dog kennels are available.
- Physical examination of animals by veterinarian is regularly done.
- Health monitoring program as per FELASA guidelines is followed.
- Feed, water and bedding material analysis is done.
- Ethical efforts to avoid pain and distress to experimental animals is followed.
- Anesthesia and Euthanasia is given as per regulatory guidelines.
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
OCTOBER 01, 2024
PROTACs: Research for a life without cancer
PROTACs: Proteolysis-targeting chimeras (PROTACs) are a rapidly evolving field with promising applications in cancer, neurodegenerative diseases, and other conditions where the regulation of protein levels is crucial. PROTACs are a novel class of small molecules designed to target specific proteins for degradation by the ubiquitin-proteasome sys...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Case study: Tackling CYP 2C9 inhibition challenges
The Problem: Active compounds in a project were found to be highly potent inhibitors of CYP 2C9 The compounds selectively inhibited CYP 2C9 with IC50 values <100 nM There was no considerable inhibition of the other CYP isoforms Our Mitigation Approach: CYP 2C9 inhibition data was generated for a larger set of co...
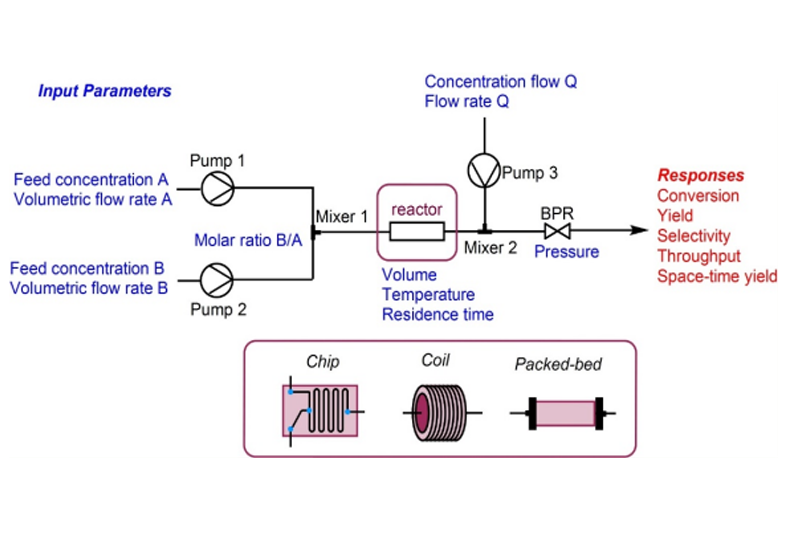
Read MoreSynthesis of Anti-covid Drug Nirmatrelvir Using Flow Chemistry
2022
Synthesis of the anti-covid therapeutic Nirmatrelvir by using flow chemistry to enhance efficiency of amide to nitrile conversion in a functionally and Stereochemically Embellished environment. ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market