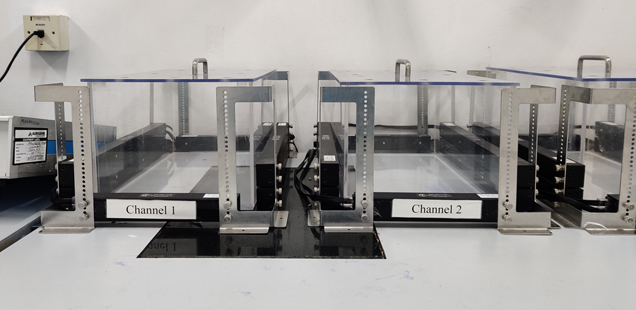
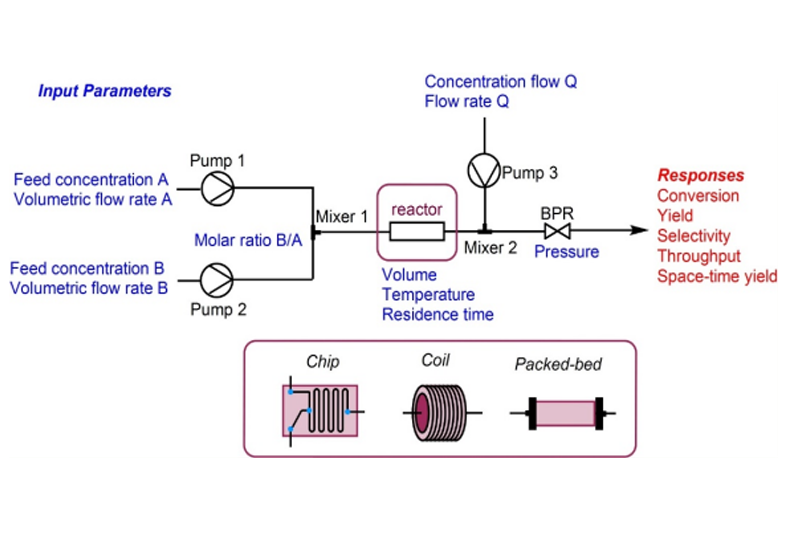
Our DMPK team is having expertise in both integrated drug discovery and standalone DMPK services with experience over 16 years. Our capabilities helped us to deliver more than 65 IDD projects. We design customized protocols and perform assays based on client or project requirements. The DMPK facility spanning an area of 10,000 sq.ft. in Bangalore and Hyderabad locations consists of state-of-the-art in vitro ADME and tissue culture labs, AAALAC accredited animal facility for in vivo PK studies in rats, mice & dogs and GLP & Non-GLP bioanalytical labs with high-end LC-MS/MS instruments.
In vitro ADME capable of high-throughput screening with best in industry turnaround times. In vivo PK studies with various established surgical models. Experience in DMPK studies and bioanalysis of small molecules, PROTACs, therapeutic peptides and biomarkers. Automated compound management and data handling systems.







Why Aurigene ADME/DMPK Services?
Turnaround time: In vitro Assays: 5 working days. In vivo PK Studies: 7 working days
Quality & accuracy
Customized protocols
Compound management and data automation
Broad panel of in vitro ADME & in vivo PK study designs
Bioanalysis of small molecules, therapeutic peptides, biomarkers & complex molecules. Delivered 200+ GLP studies
Other Services
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.
Resources
OCTOBER 01, 2024
PROTACs: Research for a life without cancer
PROTACs: Proteolysis-targeting chimeras (PROTACs) are a rapidly evolving field with promising applications in cancer, neurodegenerative diseases, and other conditions where the regulation of protein levels is crucial. PROTACs are a novel class of small molecules designed to target specific proteins for degradation by the ubiquitin-proteasome sys...
Read More
Advancement in personalized medicine and how the CRDMO industry is part of the solution
Personalized medicine is transforming the healthcare landscape by customizing treatment plans to individual patients’ unique genetic, clinical and environmental characteristics. These are effective and less invasive treatments for a wide range of conditions. Contract Research, Development and Manufacturing Organizations (CRDMOs) play an important role...
Read More
Cell Line Development
We enable development of stable and high yielding recombinant Mammalian and Microbial lines. ...
Read More
Case study: Tackling CYP 2C9 inhibition challenges
The Problem: Active compounds in a project were found to be highly potent inhibitors of CYP 2C9 The compounds selectively inhibited CYP 2C9 with IC50 values <100 nM There was no considerable inhibition of the other CYP isoforms Our Mitigation Approach: CYP 2C9 inhibition data was generated for a larger set of co...
Read MoreSynthesis of Anti-covid Drug Nirmatrelvir Using Flow Chemistry
2022
Synthesis of the anti-covid therapeutic Nirmatrelvir by using flow chemistry to enhance efficiency of amide to nitrile conversion in a functionally and Stereochemically Embellished environment. ...
Read More-
Discovery of MAP855, an Efficacious and Selective MEK1/2 Inhibitor with an ATP-Competitive Mode of Action.
2005
Mutations in MEK1/2 have been described as a resistance mechanism to BRAF/MEK inhibitor treatment. We report the discovery of a novel ATP-competitive MEK1/2 inhibitor with efficacy in wildtype (WT) and mutant MEK12 models. Starting from a HTS hit, we obtained selective, cellularly active ...
Read More -
Wang-OSO3H catalyzed green synthesis of bioactive isoindolo[2,1- a ]quinazoline-5,11–dione derivatives: An unexpected observation
2005
The sulphonic acid-functionalized Wang resin (Wang-OSO3H) was explored as a polymeric and recov- erable acidic catalyst for the synthesis of isoindolo[2,1- a ]quinazoline-5,11–dione derivatives under green conditions. Thus the Wang-OSO3H ...
Read More -
Polycyclic Aromatic Compounds: A Simple and Efficient [(n-Bu3Sn)2MO4]n Catalyzed Synthesis of Quinazolinones and Dihydroquinazolinones
2005
A novel unprecedented approach for the synthesis of various quinazolinones and dihydroquinazolinones has been using [(n-Bu3Sn)2MO4]n as a catalyst. The reaction has been screened ...
Read More
Frequently asked questions
Why is ADME important?
ADME data helps scientists assess and optimize the absorption, distribution, metabolism, and excretion of the drug/NCE early in the drug discovery process. This helps to minimize late-stage failures in in-vivo safety and efficacy studies and clinical trials.
What is ADME testing?
ADME testing involves various in-vitro and in-vivo assays used to assess the properties that determine the Absorption, Distribution, Metabolism, and Excretion (ADME) of NCEs/ drug molecules.
What is the ADME process?
The ADME process involves the characterization of a drug by using different assays to determine its absorption, distribution, metabolism, and excretion properties. This data is critical to prioritize and advance drug/ NCEs for future development.
What is DMPK in drug discovery?
DMPK is the study of the drug-like properties of new chemical entities(NCE)/drugs to understand its metabolism and pharmacokinetic profile.
What is the purpose of a PK study?
PK studies are an integral part of drug development. It helps to understand a new chemical entity (NCEs)/drug's pharmacokinetic behaviour in the body and involves the study of distribution, metabolism, clearance and bioavailability.
What is the difference between PK and PD?
Pharmacokinetics (PK) is the response of the body to the drug. Whereas Pharmacodynamics (PD) is the action of the drug on our body.
What are the recommended storage conditions for Oligonucleotides?
For a period of 6 weeks, Oligos can be stored in T10E1 buffer at 37°C and for the long term, Oligonucleotides can be dried down and stored with or without TE buffer at -20°C.
What is pharmacodynamics of a drug?
Pharmacodynamics (PD) of a drug refers to the biochemical and physiological effects caused by the drug/NCE in the body.
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market