Our early-phase formulation development services help to resolve drug delivery challenges encountered at both preclinical and clinical development stages of small molecules and peptides. We offer specific solutions to your preformulation and formulation needs through customized development strategies and designs.
Our expert team of scientists have successfully delivered projects in wide range of areas like incomplete dissolution, low solubility, low oral bioavailability and targeted drug delivery through in-depth molecular characterization, various solubilization and enabling technologies.



Our Services for Preclinical Formulation Development Include
Many hits and lead NCEs get shelved from pharmacological and toxicity screening despite high potency because of poor bioavailability or suboptimal formulation. As a leading CDMO company, we have more than a decade of experience in helping our clients overcome these challenges by enabling preclinical and GLP toxicology studies.
Our preclinical formulation development approach considers but is not limited to
Oral formulation
- Suspension
- Solution
- Solid dispersion
- Enteric solid dispersion
- Lipid based delivery (Emulsion/SMEDDS)
- Micro/Nano-suspension
Parenteral formulation
- Solution
- Lyophilization
- Emulsion
- Nano-suspension
Oral Bioavailability Enhancement Services Include
Our formulation team has the expertise in troubleshooting complex problems by banking various oral bioavailability enhancement strategies to accelerate your drug discovery program to the next phase.
Formulation development strategies are:
Solubilization strategies
- Micellar solubilization
- Complexation
- pH modification/buffering
- Cosolvents
- Anti-precipitating agents
Enabling technologies
- Solid dispersions
- Spray drying
- Rota-evaporation
- Spray granulation technology
- Hot melt extrusion
- Particle size reduction
- Micronization (Air-jet mill)
- Nanosizing
- Lipid-based drug delivery systems
- Emulsion
- SEDDS/SMEDDS
- Lipid formulation filled in Soft gelatin capsules
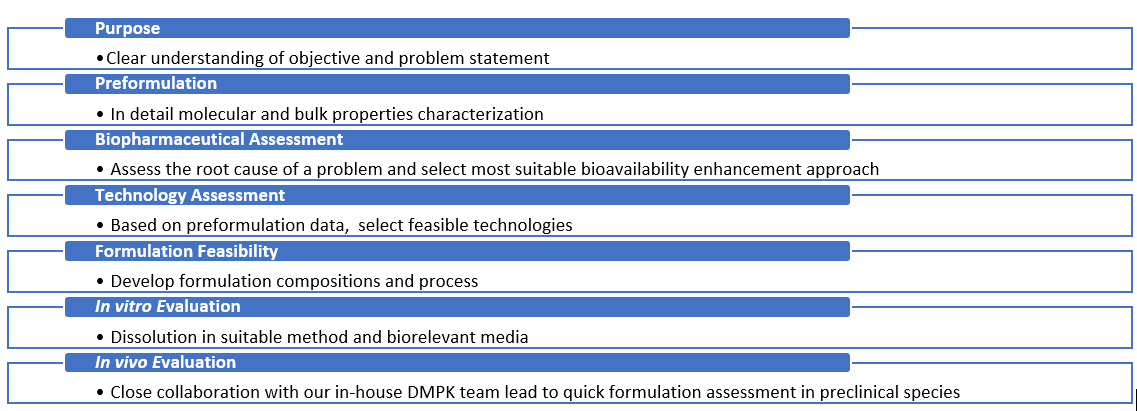
Our data-driven approach enables our partners to advance candidates and select the most optimal formulation for a specific drug development phase.
Why Aurigene Early Phase Formulation Development Services?
Services from early formulation to clinical manufacturing
Experience with advanced formulation technologies
Integration with biology services for in vitro and in vivo studies
Global accreditations
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.

Methoxy Polyethylene Glycol (m-PEGs)
Aurigene Pharmaceutical Services is a leader in the synthesis of activated MethoxyPolyethyleneGlycol(m-PEGs), With a comprehensive product range and customized services. ...
Read MoreBase mediated spirocyclization of quinazoline: one-step synthesis of spiro-isoindolinone dihydroquinazolinones
2020
A novel approach for the spiro-isoindolinone dihydroquinazolinones has been demonstrated from 2- aminobenzamide and 2-cyanomethyl benzoate in the presence of KHMDS as a base to get moderate yields. The reaction has been screened in various bases followed by solvents and a gram scale reaction has also been executed under the given conditions. Based on the controll...
Read More-
Ultrasound assisted rapid synthesis of mefenamic acid based indole derivatives under ligand free Cu-catalysis: Their pharmacological evaluation
2005
An improved and rapid synthesis of mefenamic acid based indole derivatives has been achieved via the ligand free Cu-catalyzed coupling-cyclization method under ultrasound irradiation. This simple, straightforward and inexpensive one-pot method involved the reaction of a terminal alkyne derived from mefenamic acid with 2- iodosulfanilides in the presence of CuI ...
Read More -
Formal synthesis of Cladospolide C & epi-Cladospolide C using R- (þ)-g-valerolactone as a chiral synthon
2005
The formal synthesis of Cladospolide-C and its analog is achieved by using enantiopure (R)-g evalerolactone 10. The significant points of this synthesis are the stereoselective dihydroxylation of a, bunsaturated ester 16 using Sharpless protocol, Wittig olefination of g evalerolactol 6 with triphenylphosphonium iodide salt 7, one pot selective oxidation ...
Read More -
Amberlite-15 promoted an unprecedented aza Michael rearrangement for one pot synthesis of dihydroquinazolinone compounds
2005
A new one pot multicomponent annulation strategy for the synthesis of various dihydroquinazolinone compounds has been developed using Amberlite-15 as a catalyst, giving good to moderate yields. In this reaction the substrate scope for amines and aldehydes was also investigated. The reaction has been checked on a large scale ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market