We operate 8 API manufacturing sites (6 sites in India, one site in the UK, and one site in Mexico). Each of these sites has a dedicated purpose associated with capacity, capability, desired market, and appropriate regulatory status.
Our capability footprint:
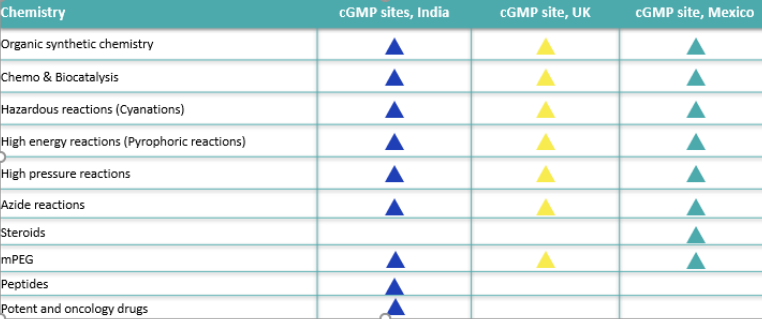


Some key sites and operational capabilities are summarized below:
CTO-III
CTO-III is one of our key sites, intended for all phases of molecules. Apart from the regular chemistry, this site can handle Cryo reactions, Hydrogenations, Suzuki coupling reactions, and many other complex reactions. In addition to the 10-production block, CTO-3 is equipped with a GMP kilo lab which enables quick scale-up to support each phase of development. The facility also comes with state-of-the art QC labs, clean rooms, downstream operations such as filtration, drying, and multi mills. This facility is inspected by all major regulatory agencies such as the FDA, WHO GMP, MHRA, COFEPRIS, and KFDA.
CTO-5
CTO-5 is used typically for tonnage volume CMO projects. This site is equipped with 16 production blocks and 12 clean rooms. There are 150+ reactors of sizes ranging from 3kl to 10 kl and above and downstream unit operations such as filters, dryers, centrifuges, multi-mills, micronizers, and ATFDs operational on the site. This site is inspected by agencies such as the FDA, PMDA, EDQM, COFEPRIS, KFDA, and MHRA.
CTO-1
CTO-I is our dedicated state-of-the-art manufacturing plant where we produce Highly Potent APIs (HPAPIs). It is audited by the US FDA, MHRA, KFDA, and TGA. The warehouse is specially designed for handling highly potent raw materials in both solid and liquid forms. The facility has different dedicated modules for large and small batch sizes, with all prerequisites to handle high potent molecules. Our reactor capacity ranges from 5 L to 1000 L.
The facility also has special unit operations across all the modules ranging from material removal into the canister, layer separation, drying, rotation evaporator, and micronization. All modules have ANFD and micronizers in SS packing isolators. The QC lab relates to Empower 3 servers and all the data related to in-process, intermediate, and complete analysis steps are transferred to SAP through LIMS software. We also offer special analytical services such as PSD, XRD, and microbiology.
CTO-SEZ
CTO-SEZ is one of our most modern automated facilities where we manufacture Intermediates, non-potent APIs, High Potent APIs (HPAPIs), and peptides. This facility is equipped with a 100L capacity peptide synthesizer and associated downstream equipment like rotavapor, and ion exchange resins. The high potent block holds 7 reactors from 160L to 1 KL capacity ranges. The facility also can handle various unit operations such as ANFD, ATFD, fluid bed dryer, multi-mill, micronizers, lyophilizers, Tangential flow filtration (TFF). This facility is audited by various agencies such as the USFDA, MFDS, and Russian Federation.
CTO-6
CTO-6 is one of our key sites operating in the Vizag cluster. This site manufactures intermediates, non-potent APIs, High Potent APIs (HPAPIs), and peptides. This facility has 12 production blocks and 18 clean rooms. This facility hosts a dedicated high-potent production block, QC, and warehousing. The peptide block is equipped with a PB-16 synthesizer and associated downstream equipment such as rotavapours, ion exchange resins, and lyophilization. CTO 6 is audited by the US FDA, WHO GMP, PMDA, EMA, Health Canada, CFDA, COFEPRIS, and MHRA.
Mexico site
Located in Cuernavaca, Mexico, this facility has over 4 decades of experience in manufacturing steroidal APIs. Our Mexico facility is equipped with dedicated, and physically separated bays as well as dedicated HVAC systems for steroidal as well as non-steroidal API production. Our reaction volume in the steroidal area ranges from 30 to 1000 gallons. This site is audited by various agencies such as the US FDA, COFEPRIS, Russian Federation, and KFDA.
Why Aurigene Sites and Capabilities Services?
Plants across continents (India, the UK, and Mexico)
20+ years of legacy, 500+ molecules worked on, 15+ commercialized
Global regulatory inspections (such as the US FDA, PMDA, EDQM, and MHRA)
cGMP scale from kg to MT
Wide range of technologies & niche reactions (peptide, steroid, and high potent)
Wide range of unit operations
Connect with our scientific experts for your drug discovery, development, and manufacturing needs
We understand that clear communication is essential to successful collaborations, and that's why we have a dedicated team that is always ready to help you. Whether you have questions about our services, want to discuss a potential partnership, or simply want to learn more about our company, we're here to help.
Our team of experts is dedicated to providing personalised solutions tailored to your unique needs. So, please don't hesitate to reach out to us. We look forward to hearing from you and helping you achieve your business goals.

Methoxy Polyethylene Glycol (m-PEGs)
Aurigene Pharmaceutical Services is a leader in the synthesis of activated MethoxyPolyethyleneGlycol(m-PEGs), With a comprehensive product range and customized services. ...
Read MoreBase mediated spirocyclization of quinazoline: one-step synthesis of spiro-isoindolinone dihydroquinazolinones
2020
A novel approach for the spiro-isoindolinone dihydroquinazolinones has been demonstrated from 2- aminobenzamide and 2-cyanomethyl benzoate in the presence of KHMDS as a base to get moderate yields. The reaction has been screened in various bases followed by solvents and a gram scale reaction has also been executed under the given conditions. Based on the controll...
Read More-
Ultrasound assisted rapid synthesis of mefenamic acid based indole derivatives under ligand free Cu-catalysis: Their pharmacological evaluation
2005
An improved and rapid synthesis of mefenamic acid based indole derivatives has been achieved via the ligand free Cu-catalyzed coupling-cyclization method under ultrasound irradiation. This simple, straightforward and inexpensive one-pot method involved the reaction of a terminal alkyne derived from mefenamic acid with 2- iodosulfanilides in the presence of CuI ...
Read More -
Formal synthesis of Cladospolide C & epi-Cladospolide C using R- (þ)-g-valerolactone as a chiral synthon
2005
The formal synthesis of Cladospolide-C and its analog is achieved by using enantiopure (R)-g evalerolactone 10. The significant points of this synthesis are the stereoselective dihydroxylation of a, bunsaturated ester 16 using Sharpless protocol, Wittig olefination of g evalerolactol 6 with triphenylphosphonium iodide salt 7, one pot selective oxidation ...
Read More -
Amberlite-15 promoted an unprecedented aza Michael rearrangement for one pot synthesis of dihydroquinazolinone compounds
2005
A new one pot multicomponent annulation strategy for the synthesis of various dihydroquinazolinone compounds has been developed using Amberlite-15 as a catalyst, giving good to moderate yields. In this reaction the substrate scope for amines and aldehydes was also investigated. The reaction has been checked on a large scale ...
Read More
You are about to leave Aurigene Pharmaceutical Services and affiliates website. Aurigene Pharmaceutical Services assumes no responsibility for the information presented on the external website or any further links from such sites. These links are presented to you only as a convenience, and the inclusion of any link does not imply endorsement by Aurigene Pharmaceutical Services.
If you wish to continue to this external website, click Proceed.


Leaving already?
Don't forget to join us at
CPHI Worldwide 2023.
October 24th-26th, 2023 | Barcelona, Spain
Get ready to accelerate your drug’s journey to the market